Method for synthesizing noble metal superfine nanowire water phase and establishing noble metal nanopore membrane by self-precipitation thereof
A precious metal, ultra-fine nanotechnology, applied in the direction of nanostructure manufacturing, nanotechnology, nanotechnology, etc., can solve the problems that have not been used to build precious metal nanoporous membranes, limitations, etc., to achieve wide applicability, save time, and low cost cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment 1: the synthesis of gold ultrafine nanowire
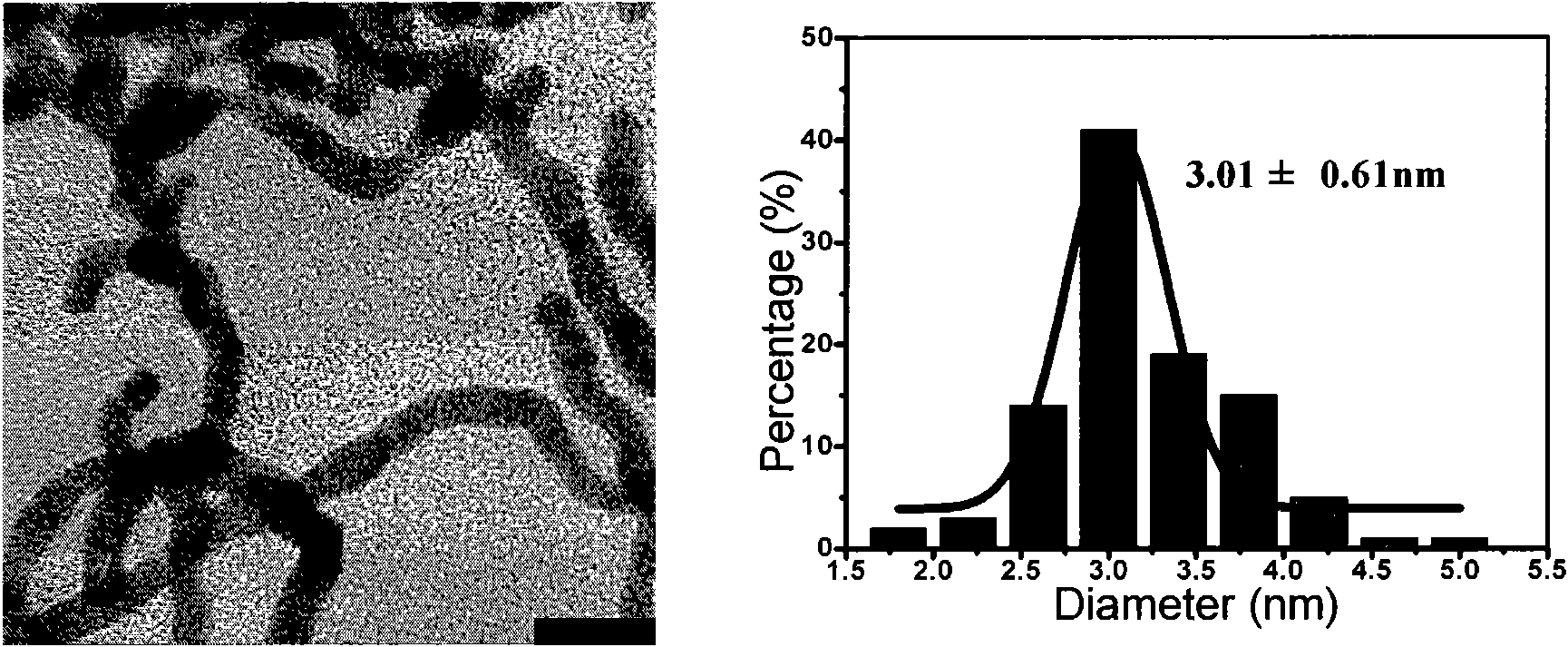
[0076] Using TX-114 as a stabilizer and structure control agent, KBH 4 as a reducing agent. 0.05mmolHAuCl 4 and 25mg TX-114 were dissolved in 47ml Millipore ultrapure water and added to a sealed 50ml Erlenmeyer flask. After stirring for five minutes at 1000rpm in an ice bath, 3ml 100mM KBH was quickly injected into the solution with a syringe 4 Solution, continue to stir for 10 seconds, the color of the solution changes from bright yellow to brown, red and finally dark gray. After the reaction is completed, add 25mg TX-114 to the solution, mix well, centrifuge at 1500rpm at 60°C for 10 minutes, discard the supernatant, and redisperse the lower cloud point phase in Millipore ultrapure water to refill the corresponding precious metal Aqueous solutions / dispersions of nanowires. Observed by transmission electron microscope (TEM, H-7500, Hitachi) and high resolution transmission electron microscope (HRTEM, JEM-2100...
Embodiment 2
[0077] Embodiment 2: Synthesis of Pt ultrafine nanowires
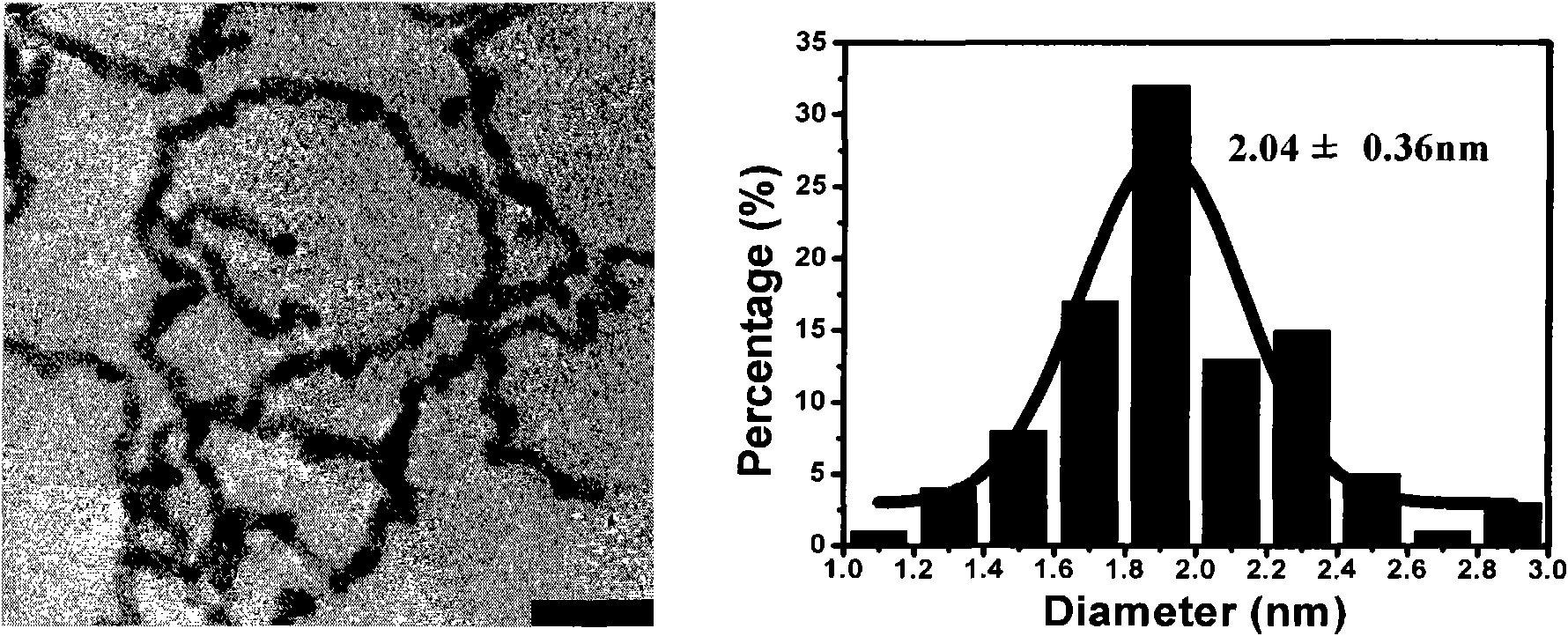
[0078] Using TX-114 as a stabilizer and structure control agent, KBH 4 as a reducing agent. 0.05mmolH 2 PtCl 6 and 25mg TX-114 were dissolved in 48ml Millipore ultrapure water and added to a sealed 50ml Erlenmeyer flask. After stirring at 1000rpm in an ice bath for five minutes, quickly inject 2ml 100mM KBH into the solution with a syringe 4 solution, continue stirring for 10 seconds. After the reaction is completed, add 25mg TX-114 to the solution, mix well, centrifuge at 1500rpm at 60°C for 10 minutes, discard the supernatant, and redisperse the lower cloud point phase in Millipore ultrapure water to obtain a new solution / dispersion liquid. Observed by transmission electron microscope (TEM, H-7500, Hitachi) and high resolution transmission electron microscope (HRTEM, JEM-2100F, JEOL), and processed by image plus software, its diameter is 2.04±0.36nm. (Such as figure 2 , the left picture is the corresponding T...
Embodiment 3
[0079] Embodiment 3: Synthesis of Pd ultrafine nanowires
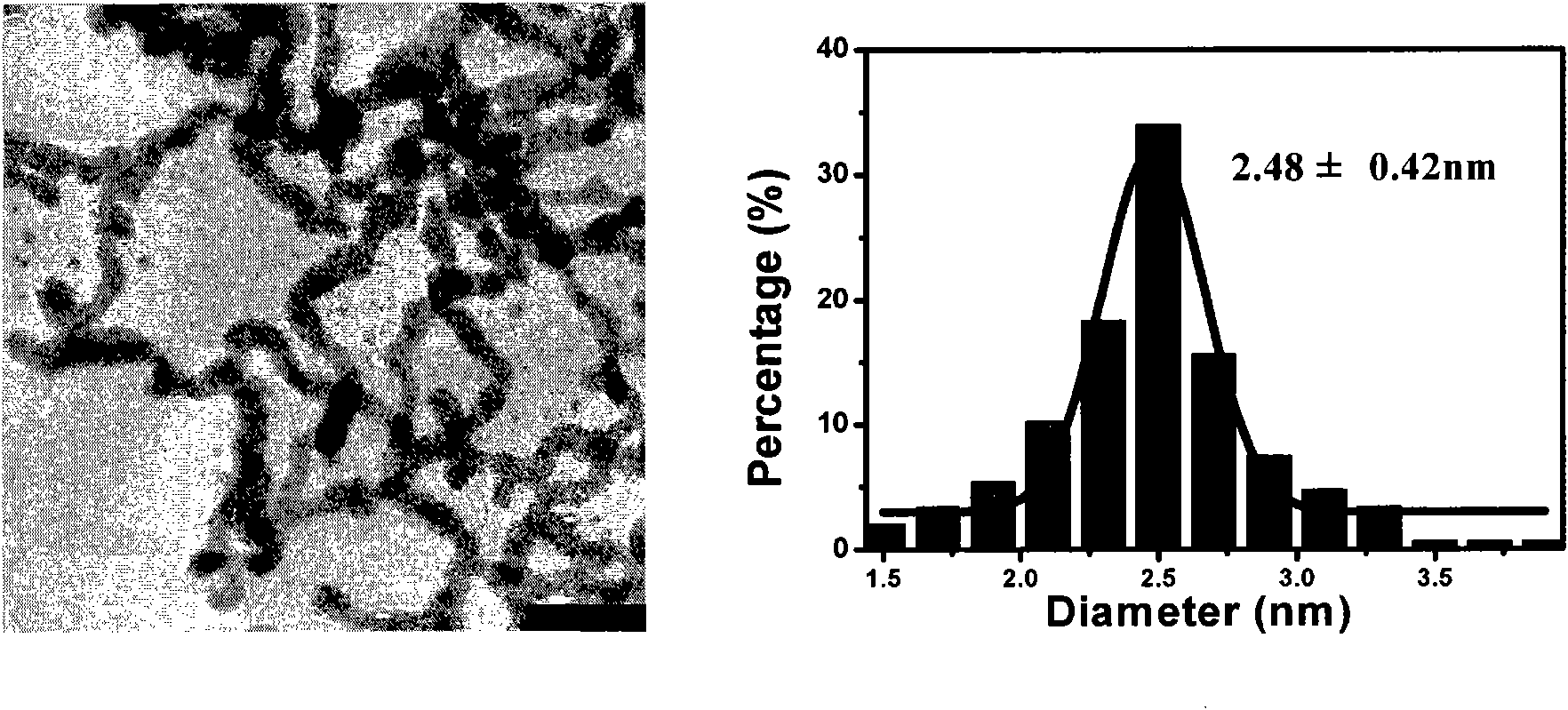
[0080] Using TX-114 as a stabilizer and structure control agent, KBH 4as a reducing agent. 0.05mmolPd(NO 3 ) 2 and 25mg TX-114 were dissolved in 49ml Millipore ultrapure water and added to a sealed 50ml Erlenmeyer flask. After stirring for five minutes at 1000rpm in an ice bath, 1ml 100mM KBH was quickly injected into the solution with a syringe 4 solution, continue stirring for 10 seconds. After the reaction is completed, add 25mg TX-114 to the solution, mix well, centrifuge at 1500rpm at 60°C for 10 minutes, discard the supernatant, and redisperse the lower cloud point phase in Millipore ultrapure water to obtain a new solution / dispersion . Observed by transmission electron microscope (TEM, H-7500, Hitachi) and high resolution transmission electron microscope (HRTEM, JEM-2100F, JEOL), and processed by image plus software, its diameter is 2.48±0.42nm. (Such as image 3 , the left picture is the corresponding TE...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com