Synthesis of several metal selenides and tellurides as semiconductor material
A metal selenide and semiconductor technology, which is applied in selenium/tellurium compounds, binary selenium/tellurium compounds, chemical instruments and methods, etc., can solve the problems of low crystallinity of products, highly toxic hydrogen selenide, and high starting temperature. , to achieve the effect of stable product quality, simple equipment and low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Weigh a certain amount of analytically pure silver nitrate, add an appropriate amount of deionized water to dissolve, transfer the clear solution to a stainless steel pressure-resistant reactor, add one-half molar amount of sodium selenite and one-fold excess of hydrazine hydrate into this To the reactor, add deionized water to 70% of the total volume, seal the reactor, and react at 100°C for 2 hours. Then it was cooled to room temperature, the reaction kettle was opened, filtered with a cloth funnel, and washed with deionized water to obtain a black powder. The product was identified as silver selenide by X-ray powder diffraction; under other conditions unchanged, the hydrazine hydrate in the above raw materials was replaced by sodium borohydride, potassium borohydride, hydroxylamine, and hydrazine sulfate, and sodium selenite was replaced by sodium borohydride, potassium borohydride, hydroxylamine, and hydrazine sulfate. After becoming selenite and ammonium selenite, the ...
Embodiment 2
[0028] Weigh a certain amount of analytically pure zinc nitrate, add an appropriate amount of deionized water to dissolve, transfer this clear solution to a stainless steel pressure-resistant reactor, add equimolar amounts of sodium selenite and an excess of 3 times hydrazine hydrate into this reactor, add Deionized water to 70% of the total volume, seal the reactor, and react at 180°C for 1 day. Then it was cooled to room temperature, the reaction kettle was opened, filtered with a cloth funnel, and washed with deionized water to obtain a green powder. The product was identified as zinc selenide by X-ray powder diffraction; under other conditions unchanged, the zinc nitrate in the above raw materials was replaced with zinc chloride and zinc sulfate, and hydrazine hydrate was replaced with sodium borohydride, potassium borohydride, and hydroxylamine. After hydrazine sulfate, sodium selenite is replaced with selenite and ammonium selenite, the product is the same; the selenium sour...
Embodiment 3
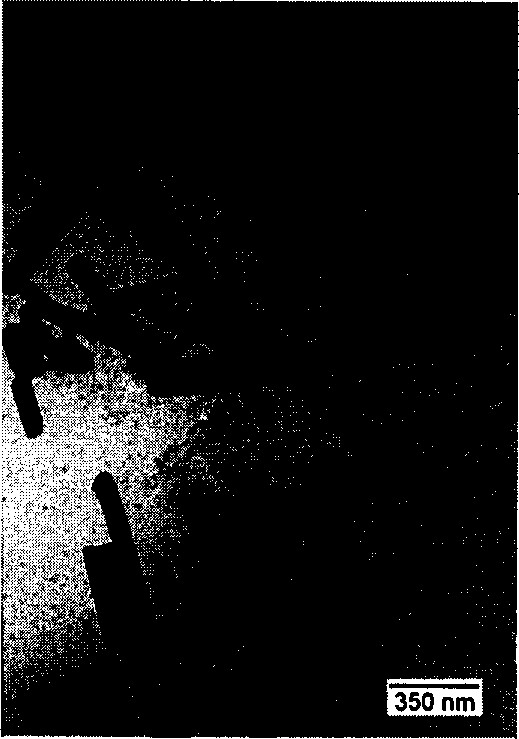
[0030] Weigh a certain amount of analytically pure cadmium nitrate, add an appropriate amount of deionized water to dissolve, transfer this clear solution to a stainless steel pressure-resistant reactor, add equimolar amounts of sodium selenite and an excess of 3 times hydrazine hydrate into this reactor, add Deionized water to 70% of the total volume, seal the reactor, and react at 100°C for 1 day. Then it was cooled to room temperature, the reaction kettle was opened, filtered with a cloth funnel, and washed with deionized water to obtain a black powder. The product was identified as cadmium selenide by X-ray powder diffraction. When other conditions remain unchanged, the reaction temperature is controlled at 100℃, 140℃, 180℃, and the preparation of cadmium selenide semiconductor materials that vary from fractal to rod shape can be controlled (see (Figure 1-Figure 3) . Replace the cadmium nitrate in the above raw materials with cadmium chloride and cadmium sulfate, replace hydra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com