Production method for preparing epsilon-caprolactone solution by using novel dehydrating agent
A production method and dehydrating agent technology, applied in the direction of preparation of peroxide compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of unstable performance, low degree of polymerization, low degree of polymerization, etc., and achieve good social benefits and Economic benefits, the effect of increasing productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1. Preparation of peracetic acid solution:
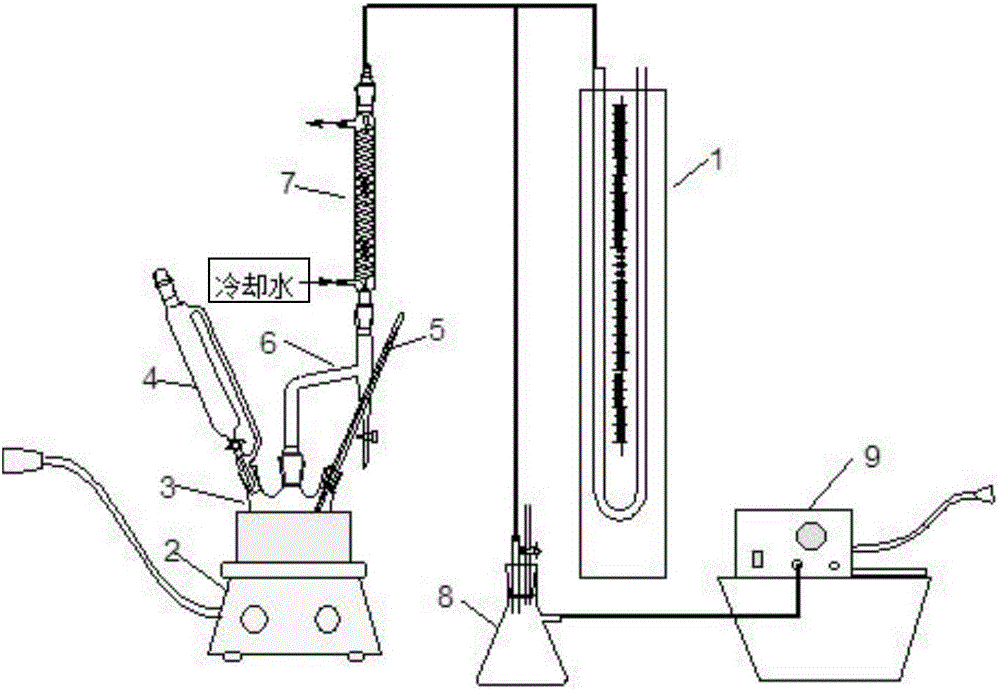
[0024] First put 2gMg / Sn / W composite oxide catalyst, 96g analytical pure acetic acid, 100g analytical pure butyl acetate, and 8 drops of analytical pure 2-methylpyridine into a three-necked flask with magnetic stirring and a reflux condenser. Stir this solution at 10-20kPa (absolute pressure), then heat it to about 56°C with a water bath, and slowly drop 54.4g of 50% (weight) hydrogen peroxide aqueous solution through a constant pressure funnel into the three-necked flask . Control the temperature of the mixed solution to 50-60°C, reflux the organic phase of the heterogeneous azeotrope condensed by the low-temperature system in the reflux condenser to the three-necked flask, and drain the water phase continuously from the water separator. Acetic acid and hydrogen peroxide reacted until the water phase was basically no longer separated in the water trap, and then the second step of the reaction was started. From the time of add...
Embodiment 2
[0031] 1. Preparation of peracetic acid solution:
[0032] First put 2gMg / Sn / W composite oxide catalyst, 96g acetic acid, 100g cyclohexane, 8 drops of 2-methylpyridine into a three-necked flask with magnetic stirring and a reflux condenser. The temperature is about 10-20kPa ( (Absolute pressure) Stir this solution, and then heat it to about 56°C with a water bath, and slowly drop 54.4 g of 50% (weight) hydrogen peroxide aqueous solution through a constant pressure funnel into the three-necked flask. The temperature of the mixed solution is controlled to be about 50-60°C, the organic phase of the heterogeneous azeotrope condensed by the low-temperature system in the reflux condenser is refluxed to the three-necked flask, and the water phase is continuously drained from the water separator. Acetic acid and hydrogen peroxide reacted until the water phase was basically no longer separated in the water trap, and then the second step of the reaction was started. From the time of addin...
Embodiment 3
[0038] 1. Preparation of peracetic acid solution:
[0039] First put 2gMg / Sn / W composite oxide catalyst, 96g acetic acid, 100g ethyl propionate, 8 drops of 2-methylpyridine into a three-necked flask with magnetic stirring and a reflux condenser, at about 10-20kPa (Absolute pressure) Stir this solution, and then heat it to about 56°C with a water bath, and slowly drop a 50% (weight) hydrogen peroxide aqueous solution with a total amount of 54.4 g into the three-necked flask through a constant pressure funnel. The temperature of the mixed solution is controlled to be about 50-60°C, the organic phase of the heterogeneous azeotrope condensed by the low-temperature system in the reflux condenser is refluxed to the three-necked flask, and the water phase is continuously drained from the water separator. Acetic acid and hydrogen peroxide reacted until the water phase was basically no longer separated in the water trap, and then the second step of the reaction was started. From the time...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com