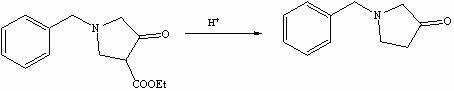
Method for preparing N-benzyl-3-pyrrolidone
A technology of pyrrolidone and benzyl, which is applied in the field of preparation of N-benzyl-3-pyrrolidone, can solve the problems of low yield and many steps, and achieves the effect of fewer synthesis steps and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
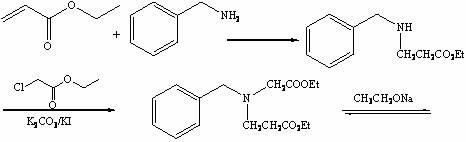
[0020] a) Synthesis of ethyl 3-benzylaminopropionate:
[0021] Add benzylamine (50.0 g, 0.467 g) into the reactor, under mechanical stirring, control the temperature ≤ 30 °C, add ethyl acrylate (82 mL, 0.766 mol) dropwise, keep the temperature at 35 °C, and stir for 15 h , gas chromatographic monitoring of the reaction raw material ethyl acrylate reaction is complete, stop stirring, distill the reaction liquid, collect 70~75 ℃ / 6mmHg fraction as excess benzylamine, collect 140~142 ℃ / 6mmHg colorless liquid as reaction product 3 -Ethyl benzylaminopropionate, yield: 96.4%;
[0022] b) Synthesis of ethyl 3-(N-ethoxycarbonylmethylene)benzylaminopropionate:
[0023] To the reactor, add ethyl 3-benzylaminopropionate (51.2 g, 0.448 mol), potassium iodide (1.3 g, 7.6 mmol), potassium carbonate (71.2 g, 0.515 mol) and ethyl chloroacetate (77 mL, 0.730 mol), stirred at room temperature for 48 h, LC-Ms monitored the reaction process, filtered after the reaction, collected the filtrate, ...
Embodiment 2
[0030] a) Synthesis of ethyl 3-benzylaminopropionate:
[0031] Add benzylamine (172.3 g, 1.608 mol) into the reactor, under mechanical stirring, control the temperature ≤ 30 °C, add ethyl acrylate (337 mL, 3.168 mol) dropwise, keep the temperature at 40 °C, and stir for 16 h , gas chromatographic monitoring of the reaction raw material ethyl acrylate reaction is complete, stop stirring, distill the reaction liquid, collect 70~75 ℃ / 6mmHg fraction as excess benzylamine, collect 140~142 ℃ / 6mmHg colorless liquid as reaction product 3 - Ethyl benzylaminopropionate; Yield: 95.9%;
[0032] b) Synthesis of ethyl 3-(N-ethoxycarbonylmethylene)benzylaminopropionate:
[0033] To the reactor, add ethyl 3-benzylaminopropionate (174.3 g, 1.528 mol), potassium iodide (4.8 g, 0.029 mol), potassium carbonate (274.5 g, 1.986 mol) and ethyl chloroacetate (283 mL, 2.674 mol), stirred at room temperature for 50 h, LC-Ms monitored the reaction progress, filtered after the reaction, collected the f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com