Irinotecan or irinotecan hydrochloride lipidosome and preparation method thereof
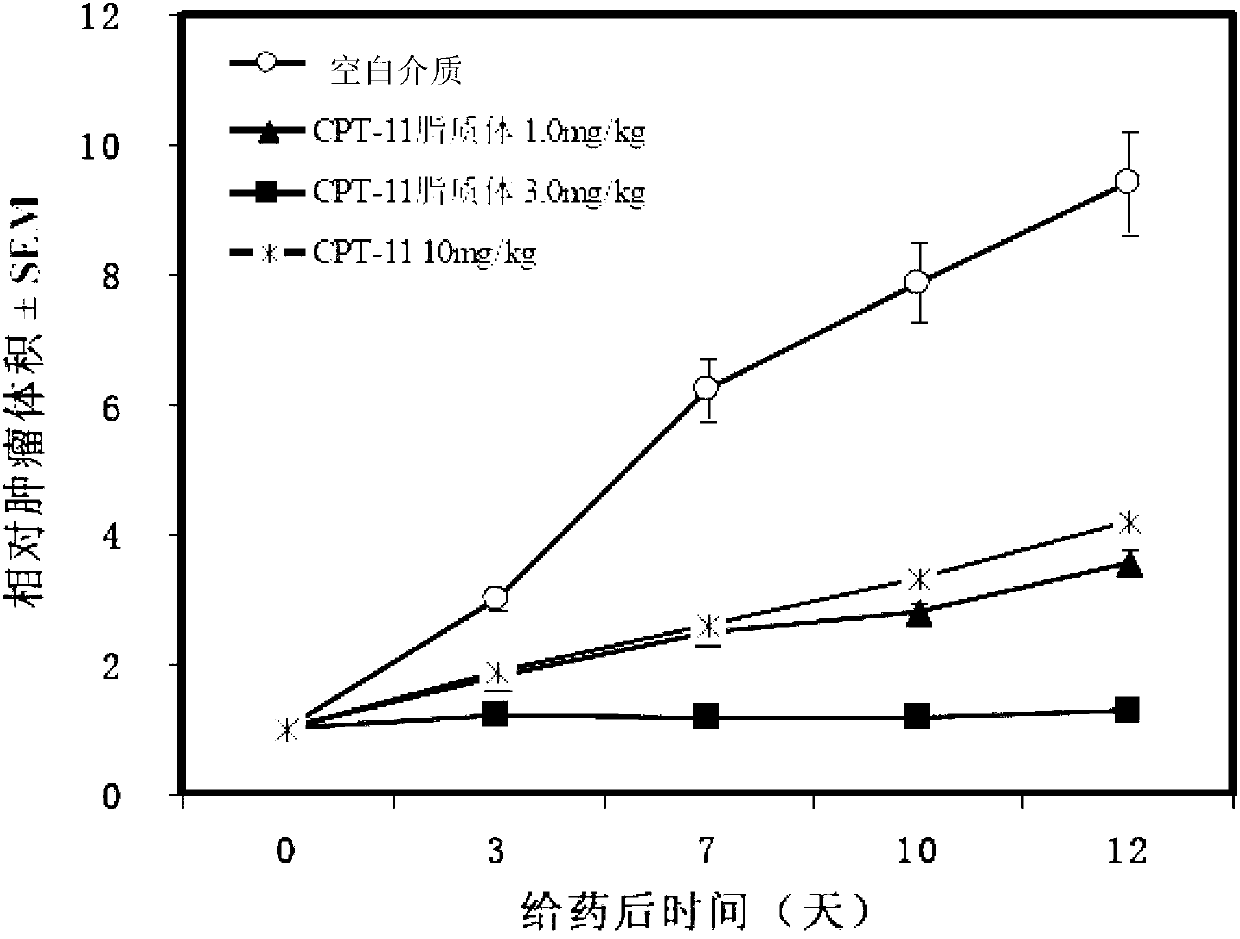
A technology of irinotecan hydrochloride and irinotecan, which is applied in liposome delivery, pharmaceutical formulation, liquid delivery, etc., can solve the problems of liposome stability and unsatisfactory particle size, and achieve toxicity reduction, inhibition The effect of increasing tumor rate and stabilizing properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] prescription
[0057]
[0058] Preparation:
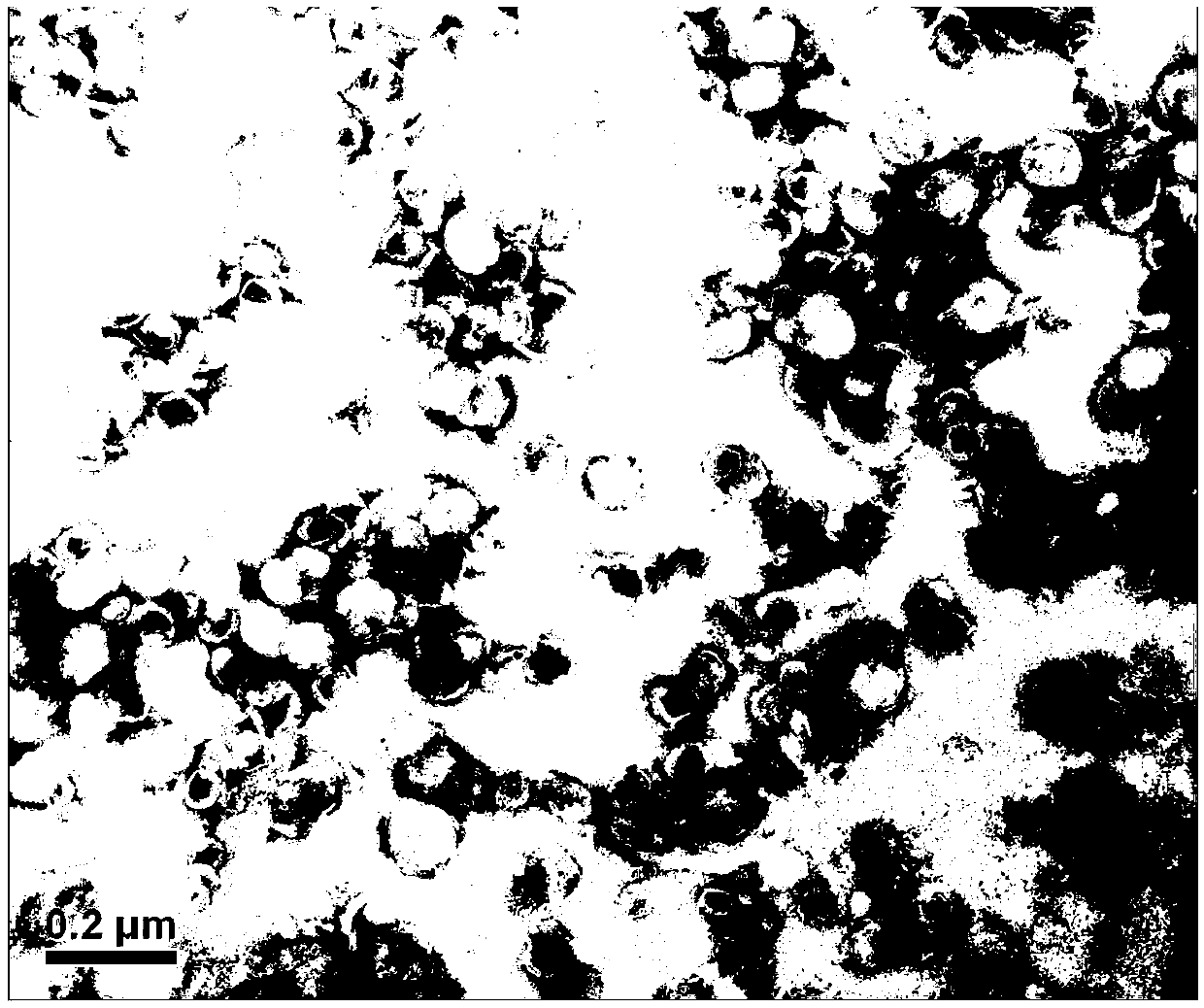
[0059] Dissolve the prescribed amount of hydrogenated soybean lecithin (HSPC) and cholesterol (CHOL) in an appropriate amount of absolute ethanol to obtain a lipid solution, mix it with 100ml of ammonium sulfate solution, and remove the ethanol under reduced pressure to obtain a crude blank liposome. Afterwards, use a high-pressure homogenizer at 1000 bar to homogenize for 5 cycles, and then extrude liposomes through extrusion equipment to control their particle size (two 0.1 μm extrusion films are laid on the extruder and extruded 5 times). Then add the prepared DSPE-PEG 2000 aqueous solution, and incubate for 20 minutes with agitation. The blank liposome was dialyzed by a tangential flow ultrafiltration device, and water for injection was supplemented continuously in the middle to obtain the blank liposome.
[0060] Prepare an aqueous solution of irinotecan hydrochloride with water for injection, add it to the above...
Embodiment 2
[0068] prescription
[0069]
[0070] Preparation:
[0071] Dissolve the hydrogenated soybean lecithin and cholesterol of the prescribed amount in an appropriate amount of absolute ethanol to obtain a lipid solution, mix it with 100ml of ammonium sulfate solution, and remove the ethanol under reduced pressure to obtain a crude blank liposome. Afterwards, use a high-pressure homogenizer at 1000 bar to homogenize for 5 cycles, and then extrude liposomes through extrusion equipment to control their particle size (extruder spreads 2 sheets of 0.1 μm extrusion film and extrudes 5 times). Then add the prepared DSPE-PEG 2000 aqueous solution, and incubate for 20 minutes with agitation. The blank liposome was dialyzed by a tangential flow ultrafiltration device, and water for injection was supplemented continuously in the middle to obtain the blank liposome.
[0072] Prepare an aqueous solution of irinotecan hydrochloride with water for injection, add it to the above-mentioned ...
Embodiment 3
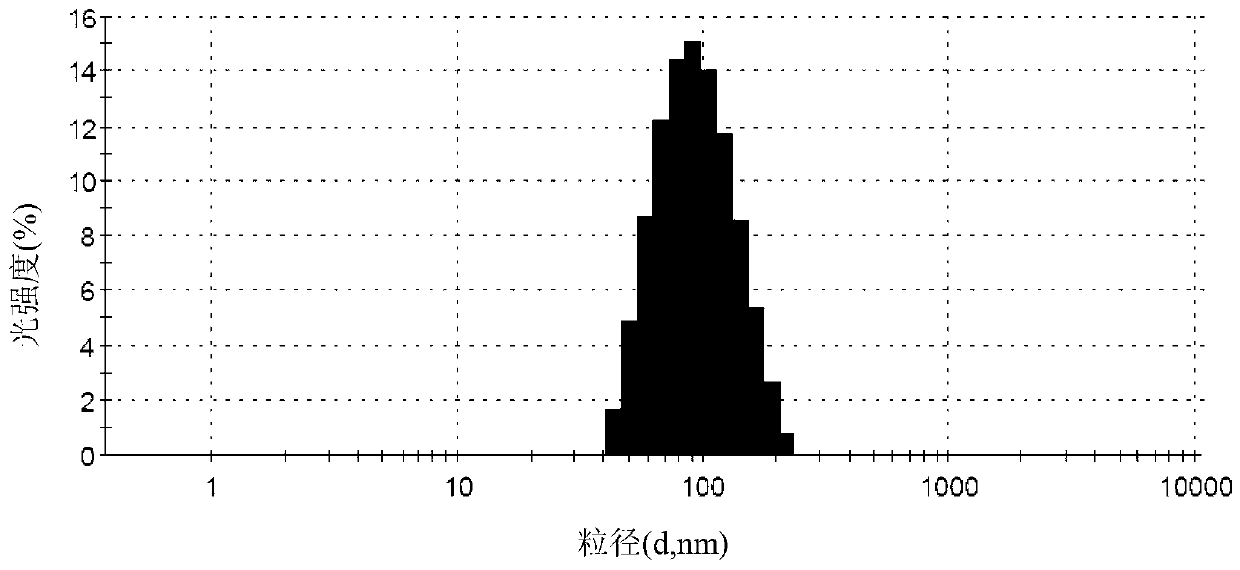
[0074] The blank liposome prescription and preparation method are the same as in Example 2, except that the weight ratios of irinotecan hydrochloride and HSPC are 1:1.5, 1:2, 1:3.5, 1:4 and 1:5 respectively. Preparation of liposomes, encapsulation efficiency and particle size of irinotecan hydrochloride liposome samples are shown in the table below.
[0075]
[0076] The results showed that when the ratio of irinotecan hydrochloride and HSPC was 1:1.5, the encapsulation efficiency was significantly reduced, and when the ratio was 1:5, the drug loading decreased significantly, and it was not suitable for preparation into clinically applied preparations. The encapsulation efficiency and drug loading are higher when the ratio is between 1:2 and 1:4.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com