Irinotecan hydrochloride injection and preparation method thereof
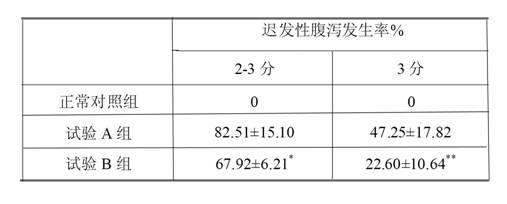
A technology of irinotecan hydrochloride and injection, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, and drug delivery, and can solve problems such as reducing adverse reactions of delayed diarrhea, safety risks of injections, and complicated preparation processes. Achieve the effect of drug dependence and treatment effect promotion, adverse reaction reduction, and simple production method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
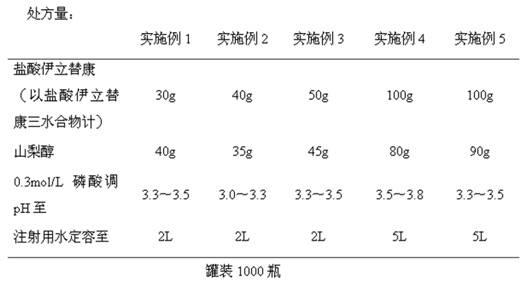
Embodiment 1-5
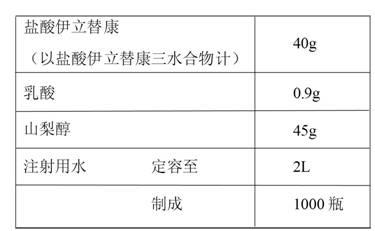
[0026] The preparation of embodiment 1-5 irinotecan hydrochloride injection
[0027]
[0028] The preparation technology of above-mentioned embodiment is as follows:
[0029] (1) Take water for injection of 75%-85% of the total volume of the prescribed amount in the batching tank, add the prescribed amount of sorbitol, stir to dissolve and mix evenly, then add the prescribed amount of irinotecan hydrochloride, at 70℃±5 Stirring and mixing under the temperature condition of ℃;
[0030] (2) Cool down the solution obtained in step (1) to below 30°C, add phosphoric acid to adjust the pH value of the solution to 3.0-3.8, and add the remaining amount of water for injection below 30°C to volume;
[0031] (3) Add activated carbon for needles accounting for 0.1% of the total weight of the solution to the solution obtained in step (2), stir for 15-45 minutes and then filter for decarbonization;
[0032] (4) The medicinal solution obtained in step (3) is filtered through one 0.45 μ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com