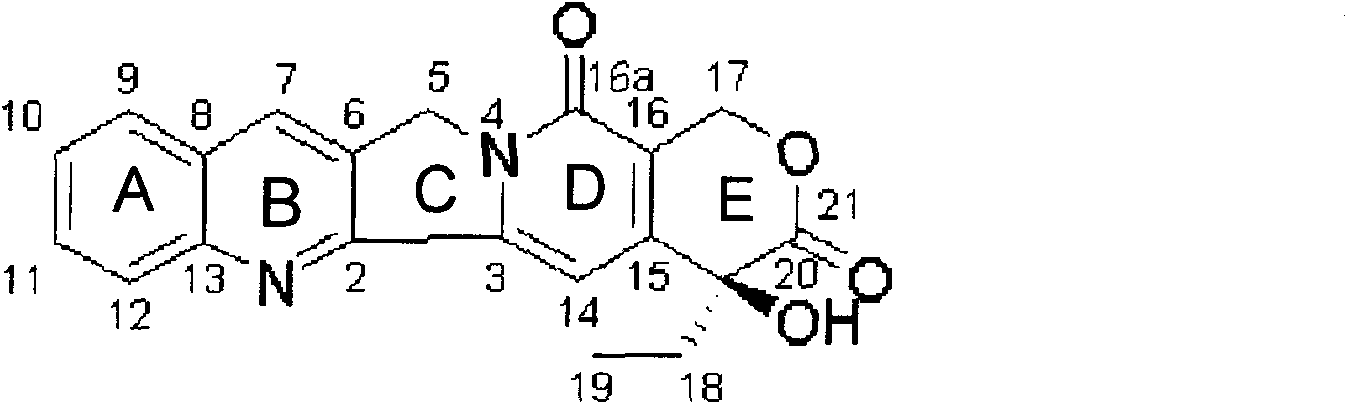
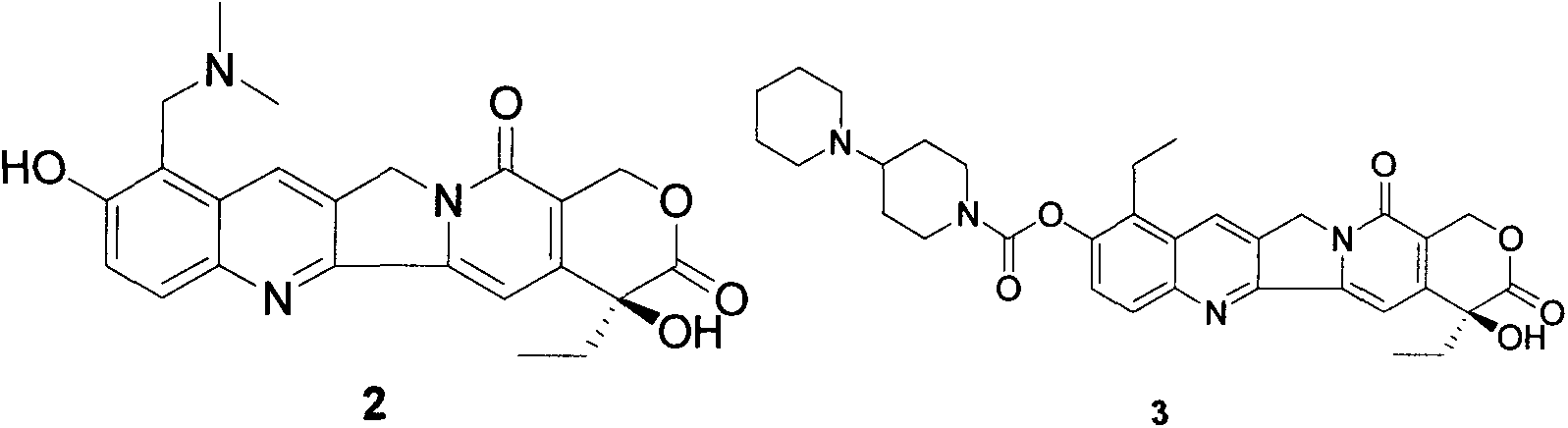
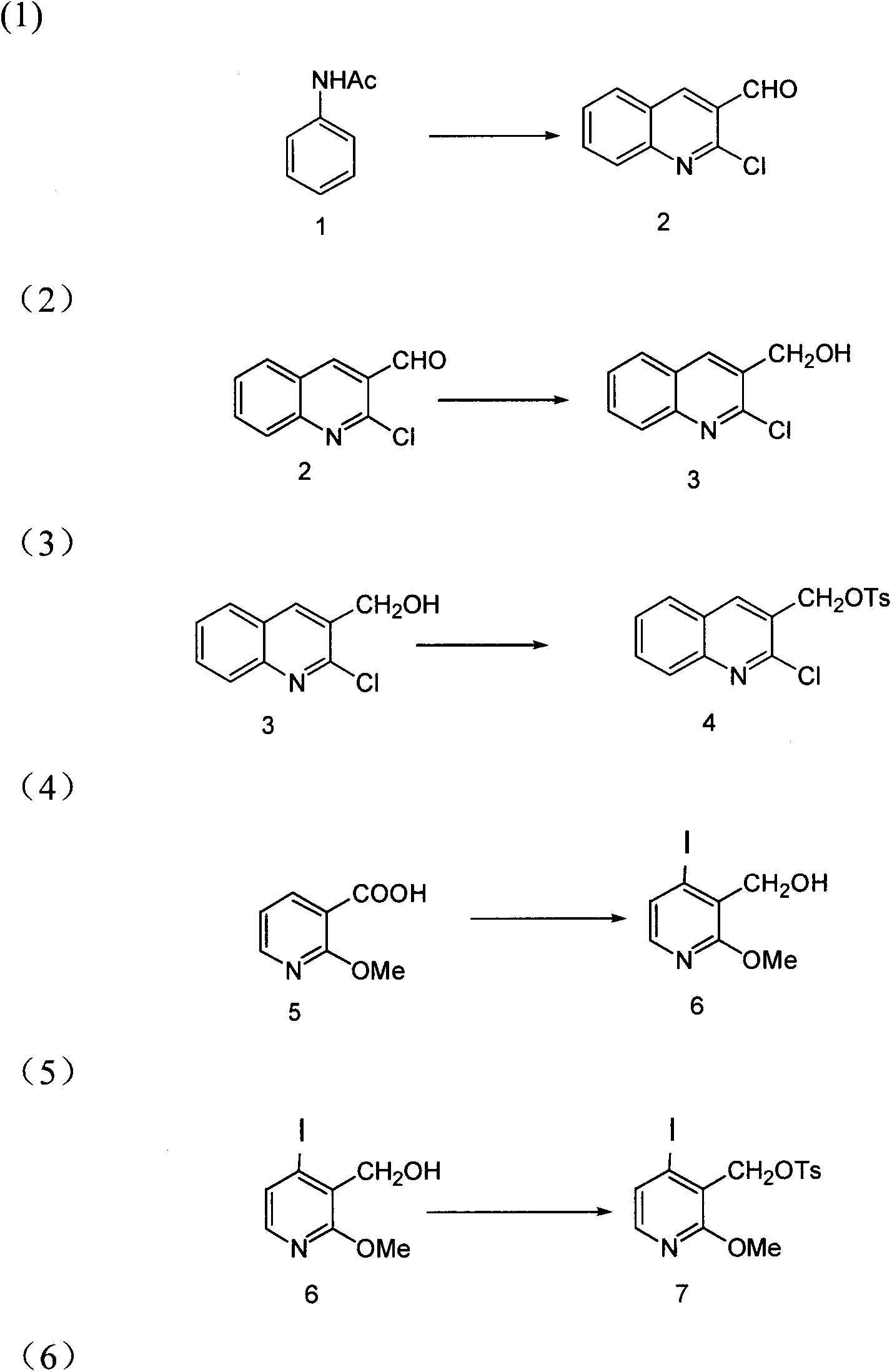
Method for synthesizing camptothecin serving as active intermediate of anti-tumor medicament
A synthetic method and technology of camptothecin, applied in the field of drug synthesis, can solve the problems of camptothecin shortage and exhaustion, extraction pollution, low efficiency, etc., and achieve the effect of low cost, efficient route and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] Add 10ml of DMF (0.13mol) to the reaction flask, cool in an ice bath, and slowly add 30ml of POCl dropwise to the reaction flask 3 (0.33mol), control the temperature not to exceed 5°C. After dropping, add compound 1 (acetanilide) (5.20g, 38.5mmol) to the reaction solution, control the temperature at 80-90°C, stir the reaction for 8-10h, and drop to At room temperature, the reaction solution was poured into a beaker filled with ice water, stirred, suction filtered, and vacuum-dried to obtain compound 2 (5.90 g, 30.8 mmol, 80%) as a pale yellow solid.
Embodiment 2
[0030]
[0031] Dissolve 5.90g of compound 2 (30.8mmol) in 50ml of THF, cool down to 0°C, and add NaBH in batches to the reaction flask 4 (1.71g, 45mmol), stirred and reacted for 3-5 hours after the addition, added ice water dropwise to the reaction flask, poured out the reaction solution, suction filtered, and dried to obtain light yellow solid compound 3 (5.84g, 30.2mmol, 98 %).
Embodiment 3
[0033]
[0034] Add compound 3 (5.84g, 30.2mmol), Et 3 N (15.18g, 0.15mol) and 100ml of THF were stirred for 0.5h, TsCl (11.54g, 60.4mmol) was added at room temperature, stirred at room temperature for 16-24h, the reaction solution was suction filtered, and the filtrate was dried with anhydrous magnesium sulfate. Suction filtration, precipitation under reduced pressure, and purification by column chromatography (EtOAc / n-hexane=1:5) gave compound 4 as a white solid (9.76 g, 28.1 mmol, 93%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com