Preparation method of irinotecan hydrochloride
A technology for irinotecan hydrochloride and salt formation, which is applied in the field of preparation of irinotecan hydrochloride, can solve the problems of complicated reaction operation and difficult purification of crude products, and achieves low isomer content and good splitting effect. , mild reaction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
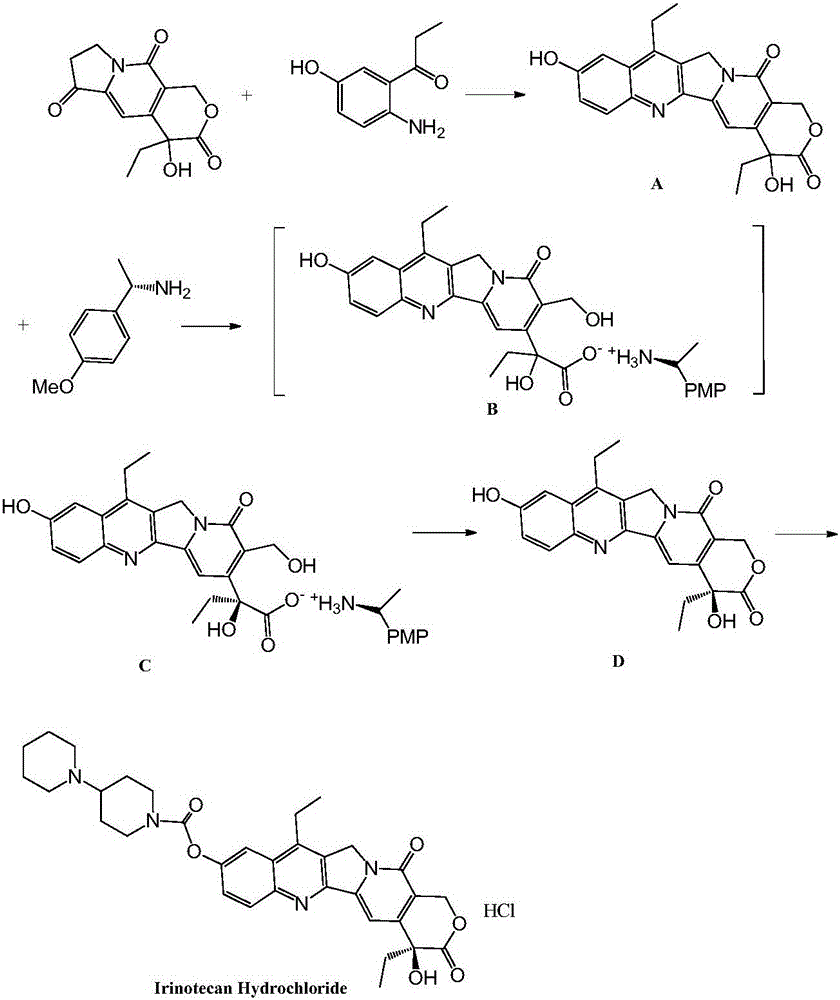
[0052] A preparation method of irinotecan hydrochloride. figure 1 It is the chemical reaction synthesis route diagram of the present invention, as figure 1 shown, including the following steps:
[0053] Add 4-ethyl-7,8-dihydro-4-hydroxy-1H-pyrano[3,4-F]indolizine-3,6,10(4H)-trione (26.3g ), toluene (260ml), stirred and dissolved. Weigh 1-(2-amino-5-hydroxyphenyl)-1-propanone (18.1 g) and p-toluenesulfonic acid (12.2 g) into the solution, and replace with nitrogen. The reaction solution was heated to slight reflux, and the reaction was maintained for 4 hours. TLC detected that the raw material was completely consumed, and the reaction solution was cooled to room temperature, washed with saturated brine (3×100ml) to pH=6-8. The organic phase was taken, dried with anhydrous sodium sulfate (20 g), concentrated under reduced pressure until no liquid dripped out. The solid compound A was obtained, weighing: 42.3 g.
[0054]The solid compound A (42.3 g) obtained in the previous...
Embodiment 2
[0058] Add 4-ethyl-7,8-dihydro-4-hydroxyl-1H-pyrano[3,4-F]indolizine-3,6,10(4H)-trione (131.5g ), toluene (1.3L), stirred to dissolve. Weigh 1-(2-amino-5-hydroxyphenyl)-1-propanone (92.1 g) and p-toluenesulfonic acid (60 g) into the solution, and replace with nitrogen. The reaction solution was heated to slight reflux, and the reaction was maintained for 4 hours. TLC detected that the raw materials were completely consumed, and the reaction solution was cooled to room temperature, washed with saturated brine (3×500ml) to pH=6-8. The organic phase was taken, dried with anhydrous sodium sulfate (100 g), and concentrated under reduced pressure until no liquid dripped out. The solid compound A was obtained, dried under vacuum at 25°C for 4 hours and weighed: 206.4g.
[0059] The solid compound A (206.4 g) obtained in the previous step was dissolved in 1.0 L of methanol, and added to a 5 L reaction flask. Weigh lithium hydroxide (13g), dissolve it in 1.0L pure water, add lithiu...
Embodiment 3
[0063] Add 4-ethyl-7,8-dihydro-4-hydroxyl-1H-pyrano[3,4-F]indolizine-3,6,10(4H)-trione (263g) into the reaction flask , toluene (2.6L), stirred to dissolve. Weigh 1-(2-amino-5-hydroxyphenyl)-1-propanone (190 g) and p-toluenesulfonic acid (122 g) into the solution, and replace with nitrogen. The reaction solution was heated to slight reflux, and the reaction was maintained for 5 hours. TLC detected that the starting material was completely consumed, and the reaction solution was cooled to room temperature, washed with saturated brine (3×1.0 L) to pH=6-8. The organic phase was taken, dried with anhydrous sodium sulfate (200 g), and concentrated under reduced pressure until no liquid dripped out. Vacuum drying at 30°C for 5 hours yielded Compound A as a solid, weighing 422 g.
[0064] The solid compound A (422 g) obtained in the previous step was dissolved in 2.0 L of methanol and added to a 10 L reaction flask. Weigh lithium hydroxide (26g), dissolve it in 2.0L pure water, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com