Preparation method of irinotecan hydrochloride injection
A technology of irinotecan hydrochloride and injection, which is applied in the field of medicine, can solve the problems such as easy precipitation of irinotecan hydrochloride, achieve stable product quality, avoid the reduction of curative effect, and have good safety effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Comparative example 1 prescription quantity:
[0032] Element Dosage (200ml prescription) Irinotecan hydrochloride (calculated as trihydrate) 4.0g Sorbitol 9.0g phosphoric acid Appropriate amount, adjust the pH value to 3.0-3.8 Water for Injection Dilute to 200ml
[0033] Remarks: Use 2ml brown vials to fill 100 vials.
[0034] The preparation process takes 75%-85% of the full amount of water for injection at room temperature, sequentially adds the prescribed amount of sorbitol and irinotecan hydrochloride, heats and stirs at 70°C ± 5°C until completely dissolved, and cools to room temperature. Adjust the pH of the medicinal solution to 3.0-3.8 with phosphoric acid, and add water for injection to make up the volume. Filter through a 0.22μm microporous membrane, fill, stopper and cap. Sterilize at 121°C for 15 minutes.
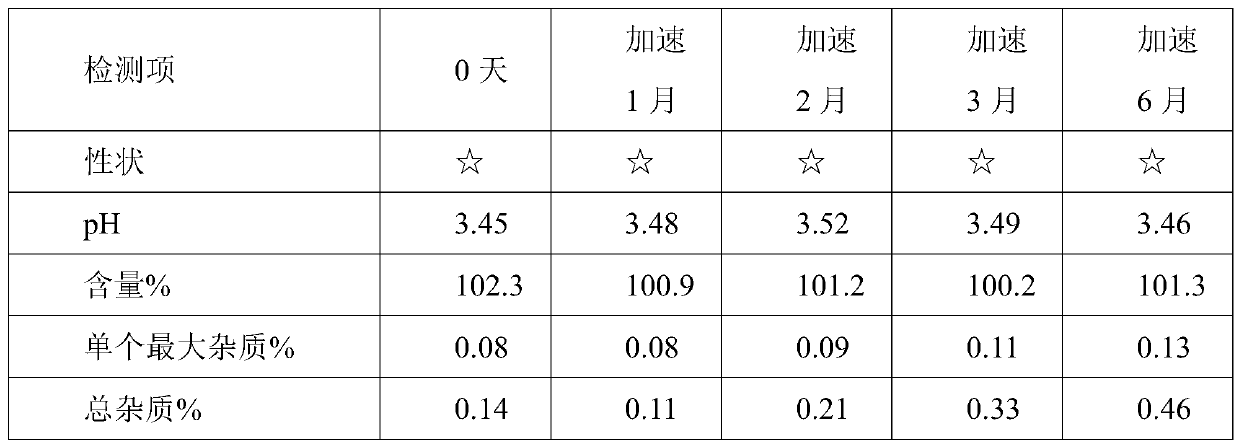
[0035] Using HPLC detection, accelerated test data are as follows:
[0036]
[0037] Remarks: 1) ☆ stand...
Embodiment 2
[0053] Take 60% of the full amount of water for injection at room temperature, add the prescribed amount of lactic acid, sorbitol, and irinotecan hydrochloride in sequence, stir and heat to 70°C, and cool to room temperature after all are dissolved. Use a pH regulator to adjust the pH of the medicinal solution to 3.0-4.0, and add water for injection to make up the volume. Filter through a 0.22μm microporous membrane, fill, stopper and cap. Sterilize at 121°C for 15 minutes.
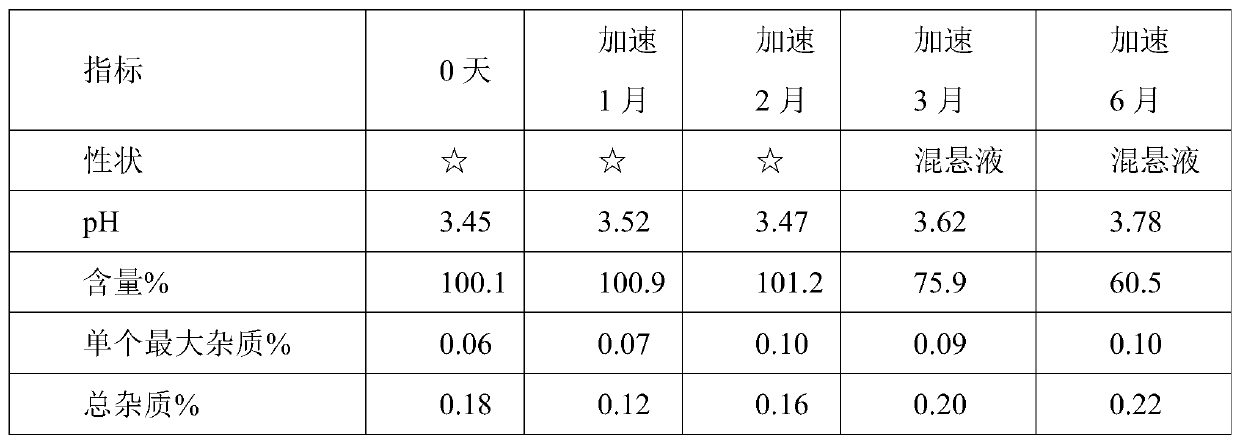
[0054] Using HPLC detection, accelerated test data are as follows:
[0055]
[0056]
[0057] Remarks: ☆ means light yellow clear liquid.
Embodiment 3
[0059] Take 50% of the full amount of water for injection at room temperature, add the prescribed amount of lactic acid, sorbitol, and irinotecan hydrochloride in sequence, stir and heat to 80°C, and cool to room temperature after completely dissolving. Use a pH regulator to adjust the pH of the medicinal solution to 3.0-4.0, and add water for injection to make up the volume. Filter through a 0.22μm microporous membrane, fill, stopper and cap. Sterilize at 121°C for 15 minutes.
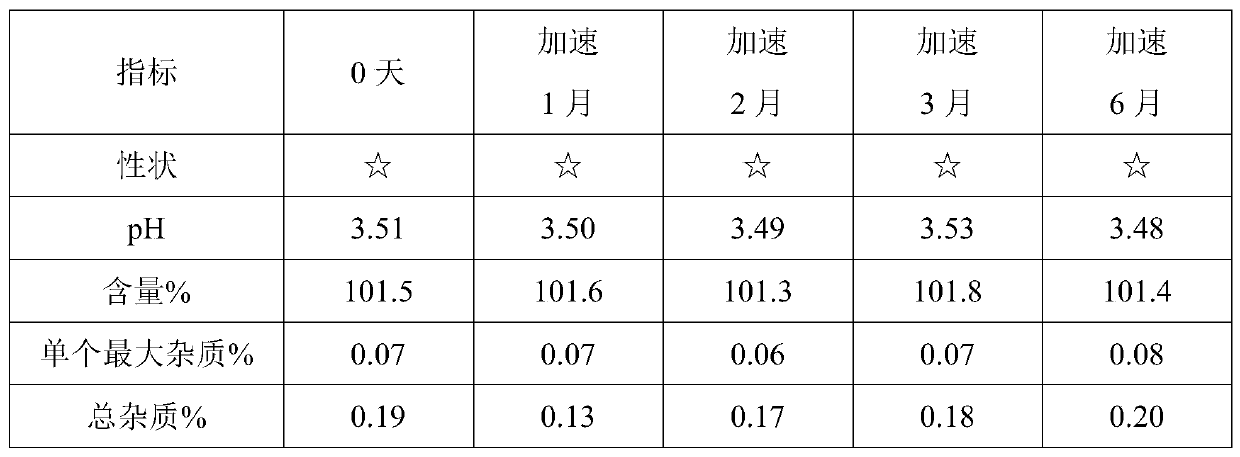
[0060] Using HPLC detection, accelerated test data are as follows:
[0061]
[0062] Remarks: ☆ means light yellow clear liquid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com