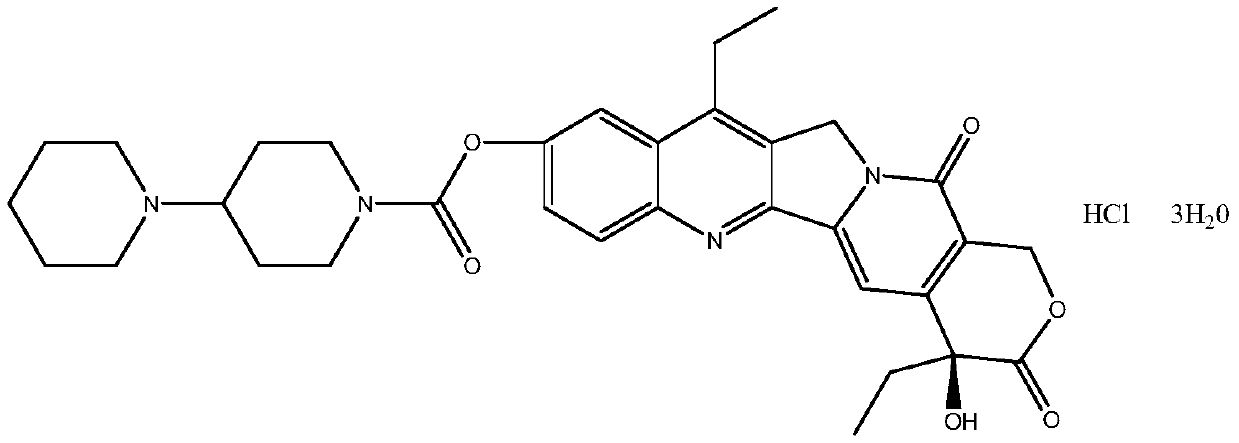
Preparation method of irinotecan hydrochloride
A technology of irinotecan hydrochloride and irinotecan, applied in the direction of organic chemistry, etc., can solve the problems of inconvenient industrial production, insoluble, poor solubility, etc., and achieve the effects of less pollution, lower production costs, and huge economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
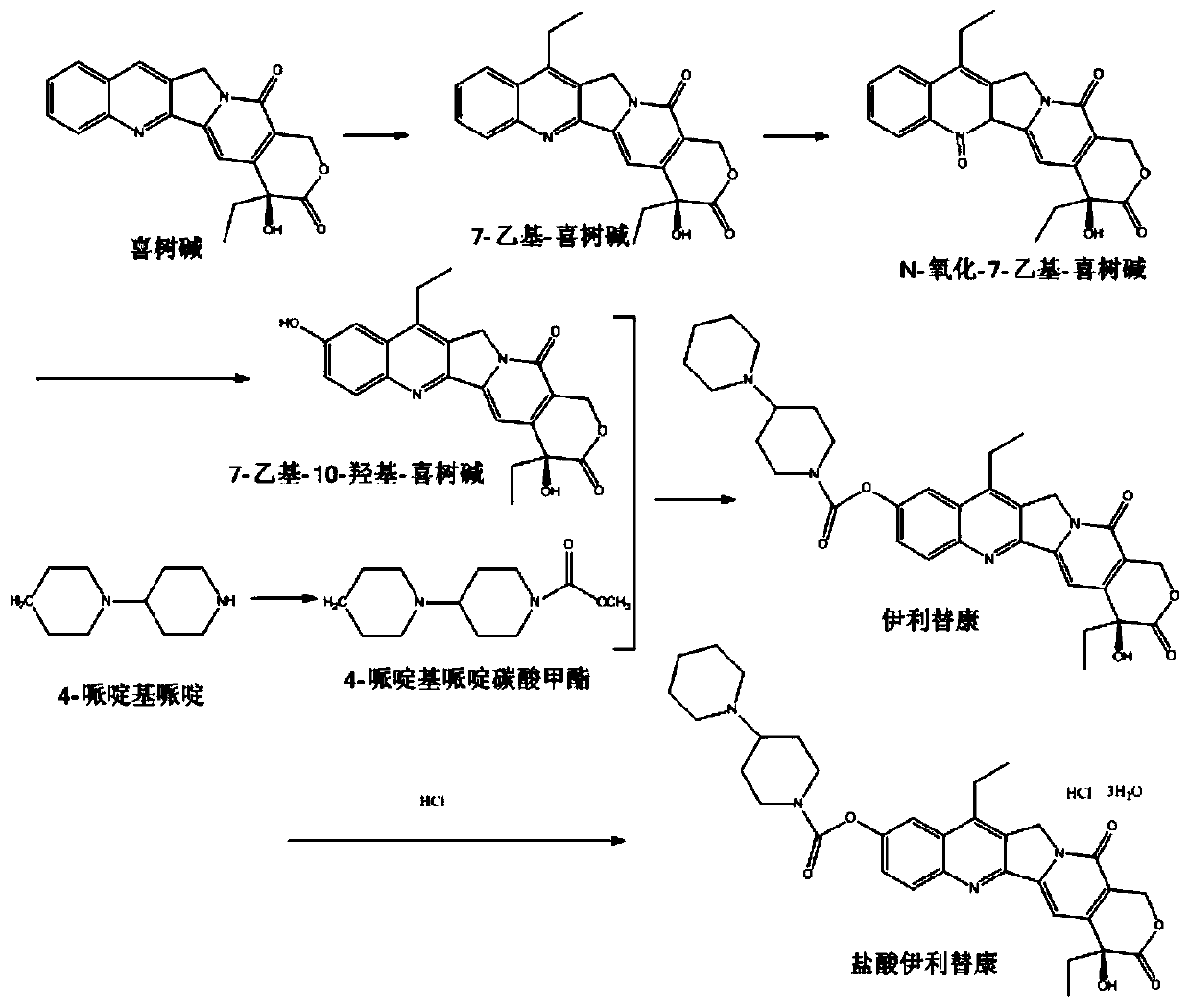
[0028] Add FeSO to a 500mL three-necked flask 4 ·7H 2 O 4.2g (15.1mmol), propionaldehyde 2.18mL (30.2mmol), water 120mL, glacial acetic acid 105mL, camptothecin 2.1g (6mmol), stir, cool to 5°C and add concentrated sulfuric acid 30mL to obtain a yellow transparent solution. Add 30% H dropwise 2 o 2 1.86mL (17.6mmol), react at 5-8°C for 15min. Pour into 500mL of ice water, adjust the pH to 8, and a large amount of yellow solid precipitates. After filtering, the filter cake was washed with a small amount of water to obtain 1.36 g of 7-ethylcamptothecin, yield: 61%.
[0029] Add 350 mL of glacial acetic acid, 30% H 2 o 2 30mL (283.9mmol), heat to 80°C, add 1.36g (3.635mmol) of 7-ethylcamptothecin after 5h, react for 3h, concentrate to about 20mL, add 500mL of ice water, let stand for 1h, suction filter, dry 1.06 g of 7-ethyl-N-oxycamptothecin product was obtained. Yield: 69.3%.
[0030] 1.06g (2.52mmol) of 7-ethyl-N-oxycamptothecin was dissolved in dioxane-acetonitrile-...
Embodiment 2
[0035] Add FeSO to a 500mL three-necked flask 4 ·7H 2 O 4.2g (15.1mmol), propionaldehyde 2.18mL (30.2mmol), water 120mL, glacial acetic acid 105mL, camptothecin 1.75g (5mmol), stir, cool to 5°C and add concentrated sulfuric acid 30mL to obtain a yellow transparent solution. Add 30% H dropwise 2 o 2 1.86mL (17.6mmol), react at 5-8°C for 15min. Pour into 500mL of ice water, adjust the pH to 8, and a large amount of yellow solid precipitates. After filtering, the filter cake was washed with a small amount of water to obtain 1.22 g of 7-ethylcamptothecin, yield: 62%.
[0036] Add 350 mL of glacial acetic acid, 30% H 2 o 2 30mL (283.9mmol), heat to 80°C, add 1.22g (3.1mmol) of 7-ethylcamptothecin after 5h, react for 3h, concentrate to about 20mL, add 500mL of ice water, let stand for 1h, suction filter, and dry 0.89 g of the product was obtained. Yield: 72%.
[0037] 0.89g (2.23mmol) of 7-ethyl-N-oxycamptothecin was dissolved in dioxane-acetonitrile-water (V:V:V=250:50...
Embodiment 3
[0042] Add FeSO to a 500mL three-necked flask 4 ·7H 2 O 4.2g (15.1mmol), propionaldehyde 2.18mL (30.2mmol), water 120mL, glacial acetic acid 105mL, camptothecin 2.45g (7mmol), stir, cool to 5°C and add concentrated sulfuric acid 30mL to obtain a yellow transparent solution. Add 30% H dropwise 2 o 2 1.86mL (17.6mmol), react at 5-8°C for 15min. Pour into 500mL of ice water, adjust the pH to 8, and a large amount of yellow solid precipitates. Filter and wash the filter cake with a small amount of water to obtain 1.66 g of 7-ethylcamptothecin, yield: 60%.
[0043] Add 350 mL of glacial acetic acid, 30% H 2 o 2 30mL (283.9mmol), heated to 80°C, 1.66g (4.27mmol) of 7-ethylcamptothecin was added after 5h, reacted for 4h, concentrated to about 20mL, added 500mL of ice water, stood for 1h, suction filtered, dried 1.07 g of product was obtained. Yield: 62%.
[0044] 1.07g (2.67mmol) of 7-ethyl-N-oxycamptothecin was dissolved in dioxane-acetonitrile-water (V:V:V=250:500:80), a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com