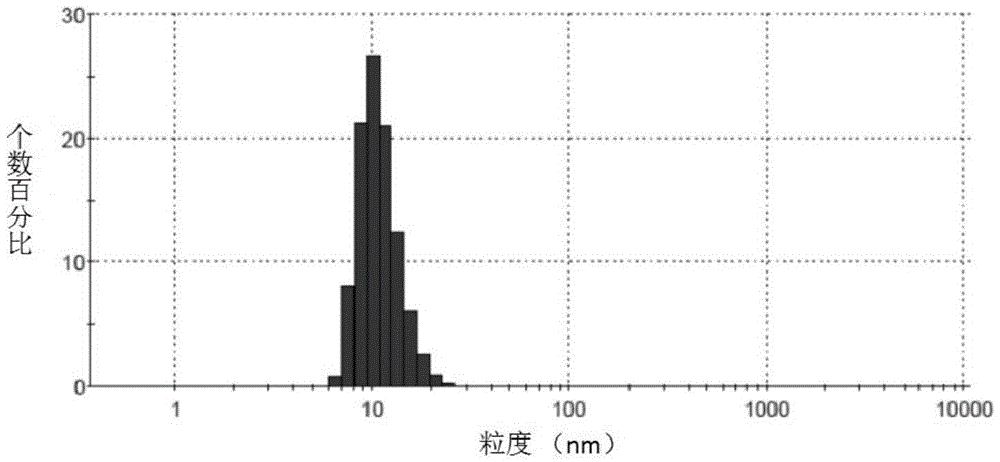
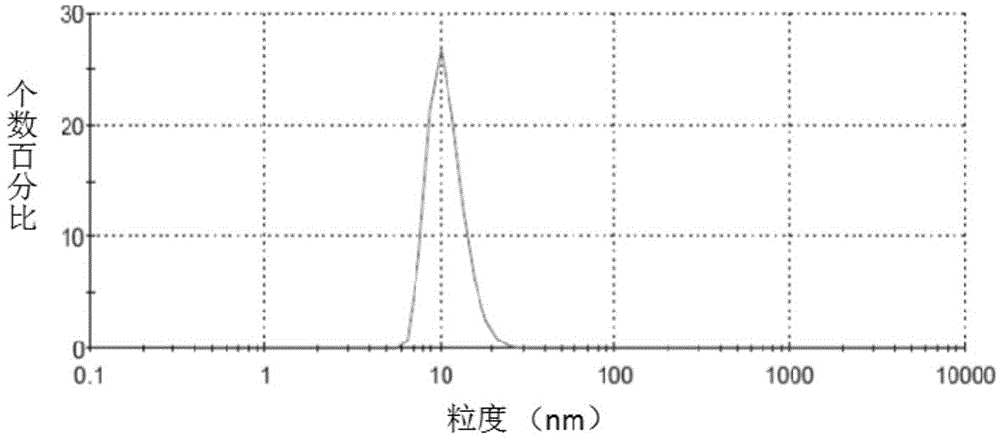
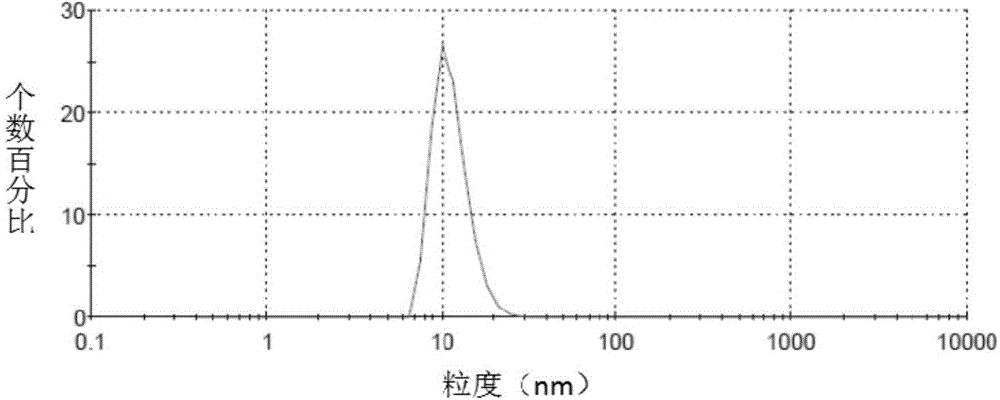
Irinotecan hydrochloride nanometer fat beam preparation and preparation method thereof
A technology of irinotecan hydrochloride and nano-lipid bundle preparation, which is applied in the field of medicine, can solve the problems of complex components, difficult to master the ratio, poor practicability, etc., and achieves the effects of simple components, simple preparation scheme and reduced toxicity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) Preparation of Irinotecan Hydrochloride Nano Lipid Beam Preparation
[0033] a) Weigh 1 g of PLGA-PEG and 10 g of water and dissolve at room temperature to obtain a solution.
[0034] b) Add 1 g of irinotecan hydrochloride to the solution prepared in step a, dissolve at room temperature, and stir thoroughly to obtain a clear solution, then filter to obtain irinotecan hydrochloride nano-lipid bundle preparation, which is protected by nitrogen gas and sealed for storage.
[0035] 2) Preparation of Irinotecan Hydrochloride Nano-Preparation
[0036] Dilute the irinotecan hydrochloride nano-lipid formulation prepared in step 1 with 20 times the volume of glucose solution to obtain the irinotecan hydrochloride nano-formulation.
Embodiment 2
[0038] 1) Preparation of Irinotecan Hydrochloride Nano Lipid Beam Preparation
[0039] a) Weigh 2g of PLGA-PEG and 20g of water, stir and dissolve in a 40°C water bath to obtain a solution.
[0040] b) Add 1 g of irinotecan hydrochloride to the solution prepared in step a, dissolve in a water bath at 50° C., stir thoroughly to obtain a clear solution, and filter to obtain irinotecan hydrochloride nano-lipid bundle preparation, which is protected by nitrogen gas and sealed for storage.
[0041] 2) Preparation of Irinotecan Hydrochloride Nano-Preparation
[0042] Dilute the irinotecan hydrochloride nano-lipid formulation prepared in step 1 with 20 times the volume of normal saline to obtain the irinotecan hydrochloride nano-formulation.
Embodiment 3
[0044] 1) Preparation of Irinotecan Hydrochloride Nano Lipid Beam Preparation
[0045] a) Weigh 2g PLGA-PEG and 30g water, stir and dissolve at room temperature to obtain a solution.
[0046] b) Add 1 g of irinotecan hydrochloride to the solution prepared in step a, dissolve in a water bath at 50° C., stir thoroughly to obtain a clear solution, and filter to obtain irinotecan hydrochloride nano-lipid bundle preparation, which is protected by nitrogen gas and sealed for storage.
[0047] 2) Preparation of Irinotecan Hydrochloride Nano-Preparation
[0048] Dilute the irinotecan hydrochloride nano-lipid formulation prepared in step 1 with 30 times the volume of glucose solution to obtain the irinotecan hydrochloride nano-formulation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com