Irinotecan hydrochloride lipidosome composition and preparation method thereof
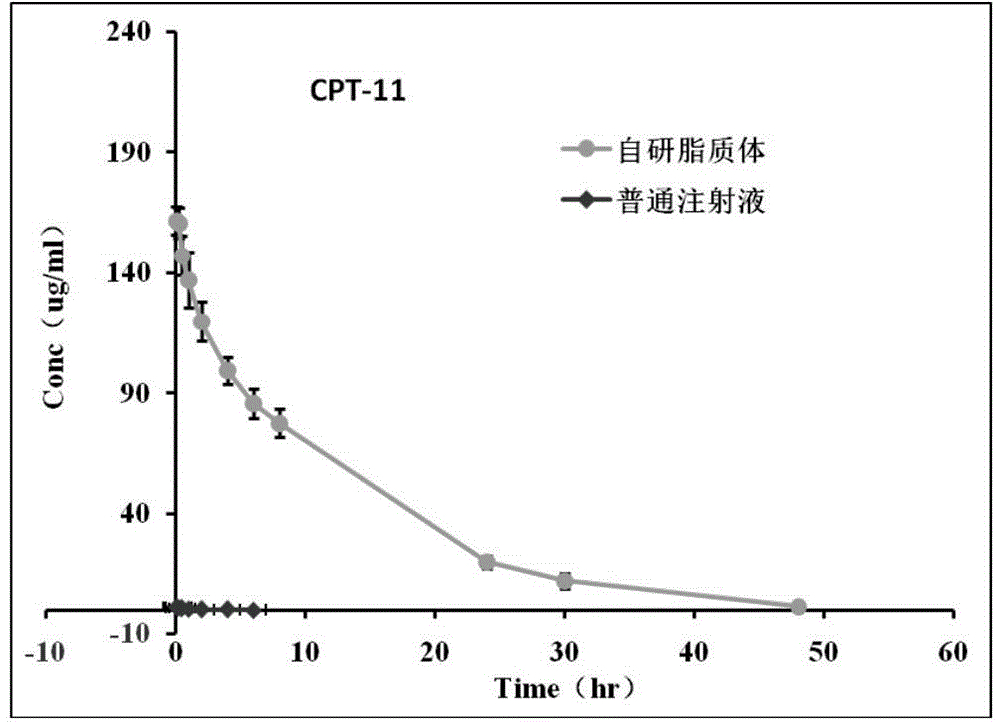
A technology of irinotecan hydrochloride and liposome composition is applied in the field of irinotecan hydrochloride liposome composition and preparation thereof, and can solve the problem of irinotecan hydrochloride yield, uncontrollable impurities, high liposome cost, The preparation process is cumbersome and other problems, and the effect of prolonging the residence time in the body, low cost and simple preparation process is achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1 The liposome excipient hydrogenated soybean phospholipid (HSPC) of the present invention, the impact of cholesterol weight ratio on irinotecan hydrochloride liposome
[0040] Table 1
[0041]
[0042] Preparation Process:
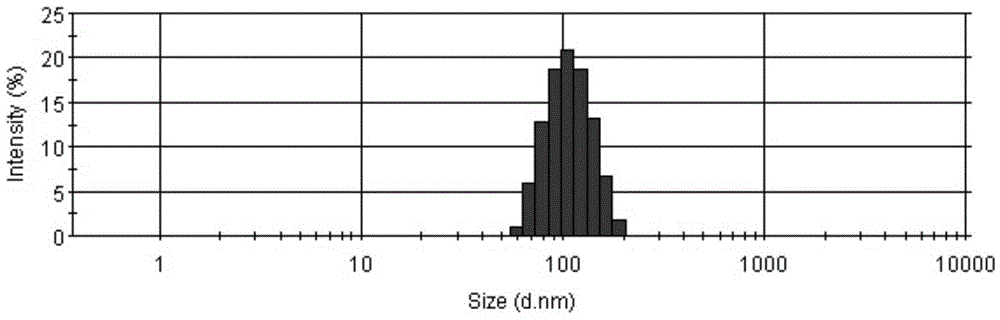
[0043]Blank liposome preparation: dissolve the hydrogenated soybean lecithin and cholesterol of prescription quantity in table 1 in 65 ℃ of dehydrated alcohols, obtain organic phase, inject it in 50-70 ℃ of ammonium sulfate solution (organic phase and ammonium sulfate solution The volume ratio is 1:5), extruded on the extruder with two 0.1μm extruded films, so that the particle size is 80-120nm. Using pH5.0-7.0 phosphate buffer as the replacement medium, the liposomes are subjected to displacement dialysis using a tangential flow ultrafiltration device (Pellicon tangential flow ultrafiltration system), so that the difference in the ammonium ion gradient of the aqueous phase inside and outside the liposome is greater than 10 3 , to ...
experiment example 2
[0052] Experimental example 2 The influence of liposome drug lipid ratio of the present invention on irinotecan hydrochloride liposome
[0053] The prescription of Table 3 is prepared irinotecan hydrochloride liposome according to the same preparation method of embodiment 1. The indexes of the prepared liposomes are shown in Table 4.
[0054] table 3
[0055]
[0056] Table 4
[0057]
[0058] From the above experimental results, it can be seen that when the drug-lipid ratio was 1:4.5, the encapsulation efficiency of irinotecan hydrochloride liposome reached more than 99%, and the phospholipid ratio in the prescription was reduced. When the drug-lipid ratio was 1:4, the encapsulation efficiency was only 1:4. 91.05%. When the drug-to-lipid ratio is 1:6, although the encapsulation efficiency also reaches 99.20%, the amount of phospholipid used is large and the cost is high. Therefore, the preferred drug-to-lipid ratio is 1:4.5.
Embodiment 3
[0059] Example 3 The influence of the preparation process of the present invention on the hemolytic phosphatidylcholine in irinotecan hydrochloride liposomes
[0060] table 5
[0061]
[0062] preparation
[0063] Sample a: According to the prescription in Table 5, the preparation process was the same as in Example 1 to obtain sample a.
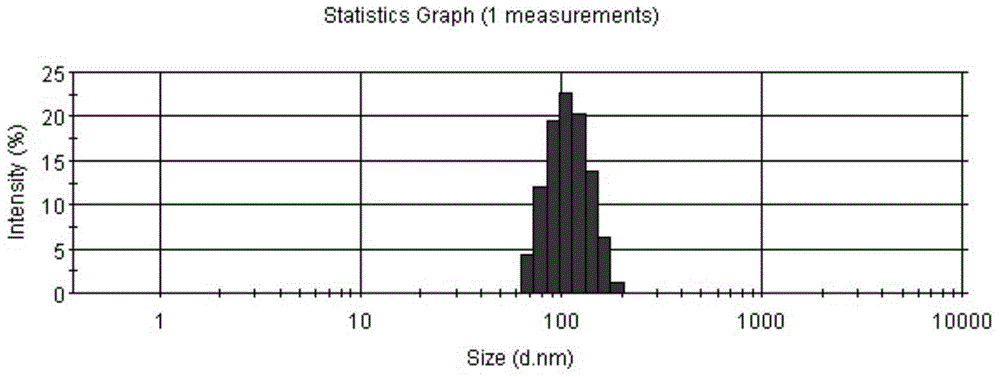
[0064] Sample b: Preparation of blank liposomes: Dissolve hydrogenated soybean lecithin and cholesterol in absolute ethanol to obtain an organic phase, which is injected into the ammonium sulfate solution of the above prescription to obtain blank liposome colostrum, which is homogenized by high pressure The machine performs high-pressure homogenization on the colostrum, 500Bar homogenization for 3 times, 1000Bar homogenization for 3 times, and then use 2 pieces of 0.1μm extrusion film to extrude on the extruder to make the particle size 80-120nm. Using pH 5.0-7.0 phosphate buffer as the replacement medium, the tangential flow ultrafiltra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com