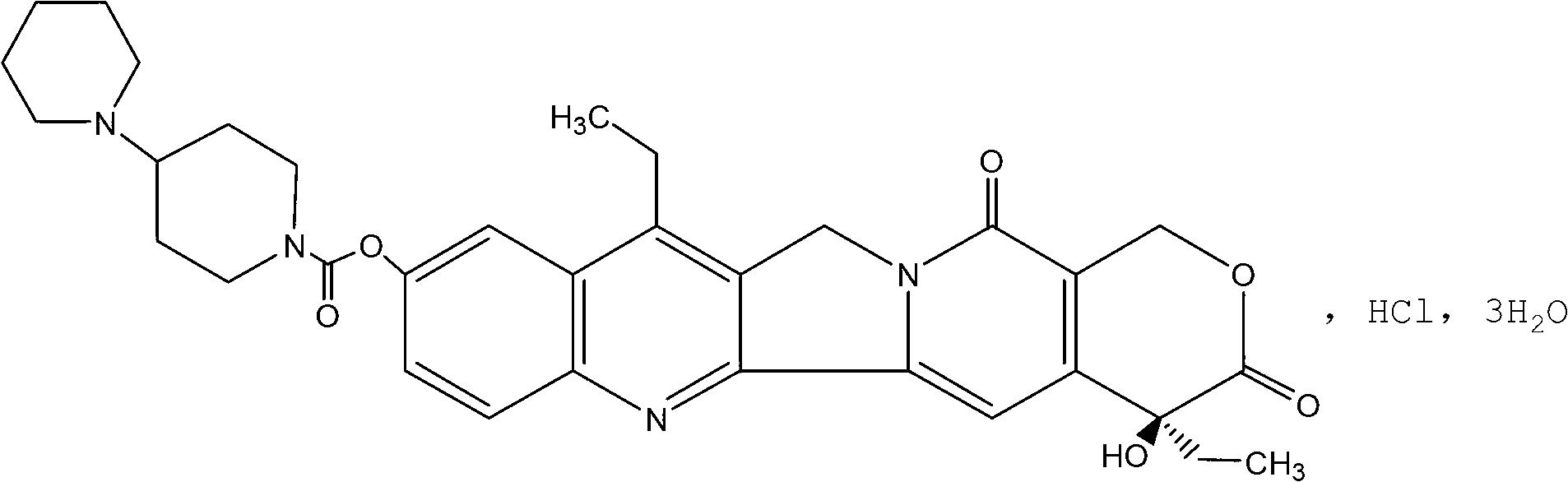
Irinotecan hydrochloride lipid nanoparticles injection
A technology of healthy lipids and nanoparticles, which is applied in the field of medicine to achieve the effects of improving stability, excellent dissolution, and good drug release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
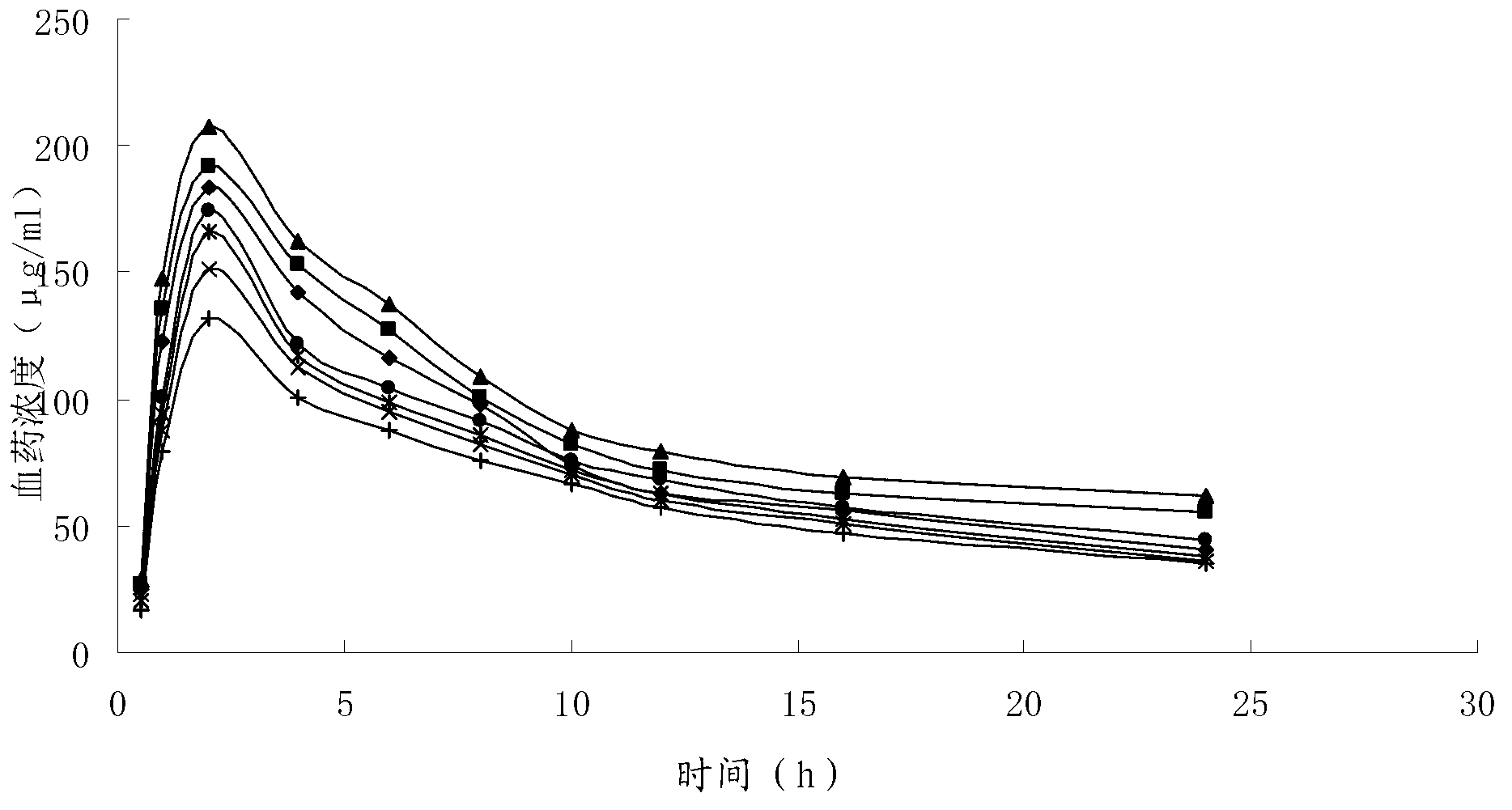
Image
Examples
Embodiment 1
[0069] Preparation of Example 1 Irinotecan Hydrochloride Lipid Nanoparticle Injection
[0070] The ingredients used are as follows:
[0071]
[0072] Adopt following production process to prepare irinotecan hydrochloride lipid nanoparticle sheet:
[0073] (1) Add 240g lecithin into 800ml pH7.0 phosphate buffer solution, heat in a constant temperature water bath at 50°C, stir to dissolve completely, then add 200ml ethanol solution in which 50g irinotecan hydrochloride and 120g stearic acid are dissolved, Stir fully to dissolve and form a mixed phase;
[0074] (2) Dissolve 320g PEG400 and 80g glycerin in 1000ml water, heat in a constant temperature water bath at 50°C, stir to dissolve, and form a water phase;
[0075] (3) Slowly add the organic phase to the stirring water phase, keep the temperature at 50°C, and continue stirring for 1 hour;
[0076] (4) Remove the organic solvent under reduced pressure to obtain translucent colostrum;
[0077] (5) Quickly add the colos...
Embodiment 2
[0079] Preparation of Example 2 Irinotecan Hydrochloride Lipid Nanoparticle Injection
[0080] The ingredients used are as follows:
[0081]
[0082]
[0083] Adopt the following production process to prepare irinotecan hydrochloride lipid nanoparticle capsules:
[0084] (1) Add 600g lecithin to 1000ml pH7.0 phosphate buffer solution, heat in a constant temperature water bath at 50°C, stir to dissolve completely, then add 300ml ethanol solution in which 100g irinotecan hydrochloride and 300g stearic acid are dissolved, Stir fully to dissolve and form a mixed phase;
[0085] (2) Dissolve 800g PEG400 and 200g glycerin in 1000ml water, heat in a constant temperature water bath at 50°C, stir to dissolve, and form a water phase;
[0086] (3) Slowly add the organic phase to the stirring water phase, keep the temperature at 50°C, and continue stirring for 1 hour;
[0087] (4) Remove the organic solvent under reduced pressure to obtain translucent colostrum;
[0088] (5) Q...
Embodiment 3
[0090] Preparation of Example 3 Irinotecan Hydrochloride Lipid Nanoparticle Injection
[0091] The ingredients used are as follows:
[0092]
[0093] Adopt following production process to prepare irinotecan hydrochloride lipid nanoparticle particle:
[0094] (1) Add 600g lecithin to 1500ml pH7.0 phosphate buffer solution, heat in a constant temperature water bath at 50°C, stir to dissolve completely, then add 300ml ethanol solution in which 100g irinotecan hydrochloride and 300g stearic acid are dissolved, Stir fully to dissolve and form a mixed phase;
[0095] (2) Dissolve 800g PEG400 and 200g glycerin in 2000ml water, heat in a constant temperature water bath at 50°C, stir to dissolve, and form a water phase;
[0096] (3) Slowly add the organic phase to the stirring water phase, keep the temperature at 50°C, and continue stirring for 1 hour;
[0097] (4) Remove the organic solvent under reduced pressure to obtain translucent colostrum;
[0098] (5) Quickly add the c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com