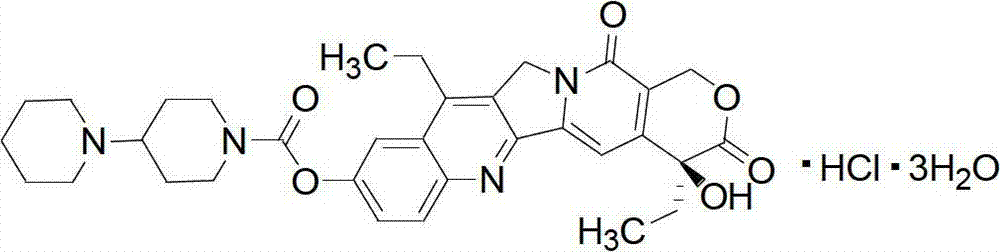
Irinotecan hydrochloride composition and preparation method thereof
A technology for irinotecan hydrochloride and its composition, which is applied in the field of irinotecan hydrochloride composition and its preparation, can solve the problems of inaccurate composition content, difficult filtration, unstable quality, etc., and achieve accurate content, high content, and guaranteed The effect of quality and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047]
[0048]
[0049] Weigh sorbitol and lactic acid into a container, add 90% water for injection, adjust the pH to 3.0~4.0 with 1mol / L sodium hydroxide solution, add irinotecan hydrochloride, dissolve and add water for injection to the full amount, then add 0.1% The activated carbon was stirred for 20 minutes and then decarburized, filtered with a 0.22 μm microporous membrane until it was clear, and the intermediate was measured, filled in a vial, plugged with a butyl rubber stopper and capped; the sample was autoclaved and leaked, Light pick up, and deliver after passing the quality inspection to be packaged, promptly obtain irinotecan hydrochloride composition.
Embodiment 2
[0051]
[0052] Weigh sorbitol and lactic acid into a container, add 90% water for injection, adjust the pH to 3.0~4.0 with sodium phosphate buffer, add irinotecan hydrochloride, dissolve and add water for injection to the full amount, then add 0.1% activated carbon After stirring for 20 minutes, decarburize, filter with a 0.22 μm microporous membrane until clear, measure the intermediate, fill it in a vial, plug it with a butyl rubber stopper and cap it; , and send it to be packaged after passing the quality inspection to obtain the irinotecan hydrochloride composition.
Embodiment 3
[0054]
[0055] Weigh sorbitol and lactic acid into a container, add 90% water for injection, adjust the pH to 3.0~4.0 with 0.05mol / L sodium hydroxide solution, add irinotecan hydrochloride, dissolve and add water for injection to the full amount, then add 0.1 % activated carbon was stirred for 20 minutes, decarburized, filtered with a 0.22 μm microporous membrane until clear, tested for the intermediate, filled in a vial, plugged with a butyl rubber stopper and capped; the sample was autoclaved and leaked , pick it up, and send it to pack after passing the quality inspection to obtain the irinotecan hydrochloride composition.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com