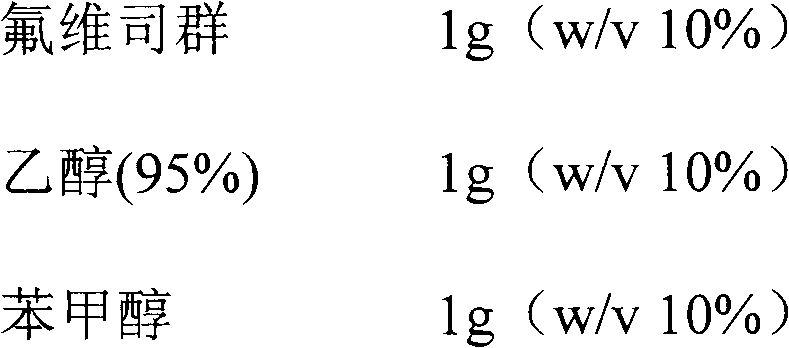Pharmaceutical composition of fulvestrant
A technology of fulvestrant and composition, which is applied in the field of pharmaceutical preparations, to achieve the effects of convenient administration, stable content, and elimination of the risk of muscle stimulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
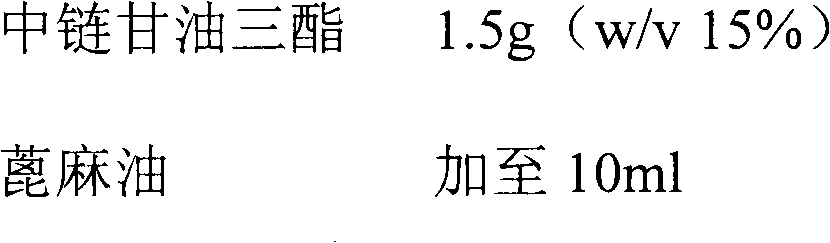
[0034] Example 1 Preparation of 100mg / ml Fulvestrant Injection
[0035]
[0036]
[0037] Preparation: Get the prescribed amount of fulvestrant, ethanol (95%), benzyl alcohol, and medium-chain triglycerides, stir and mix at room temperature until fulvestrant is completely dissolved, add castor oil to the final volume (10ml), stir and mix uniform. Filter the mixed solution through a 0.2 micron filter 1-2 times to achieve the purpose of sterilization. Under sterile conditions, dispense the sample into injection vials or pre-filled injection devices, and obtain it after visual inspection.
Embodiment 2~6
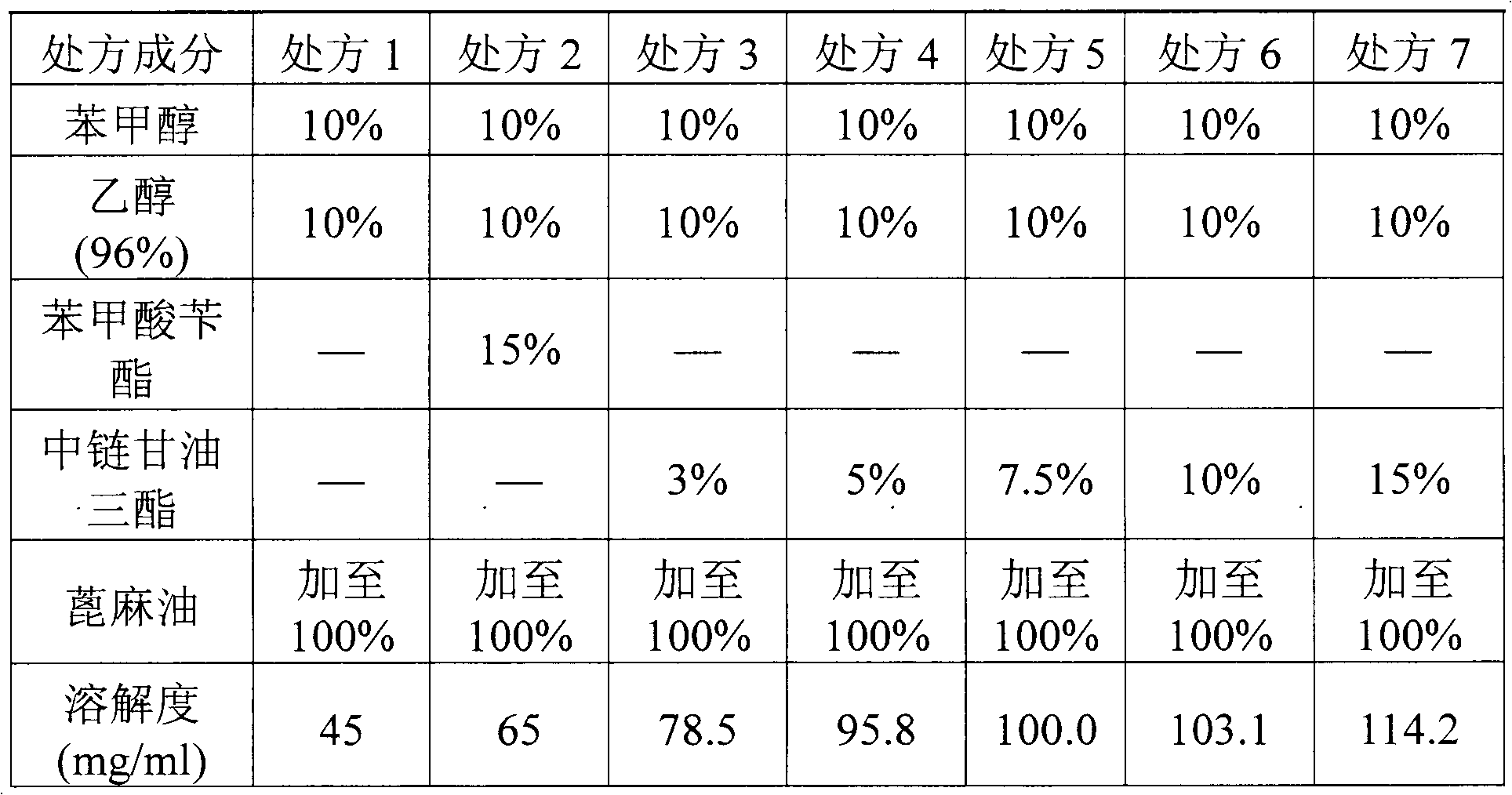
[0038] Embodiment 2~6 Preparation of Fulvestrant Injection
[0039] The dosages of fulvestrant and medium-chain triglycerides in the prescription are as shown in the table below, and the dosages of other ingredients are the same as in Example 1, and the injection is prepared according to Example 1.
[0040] Table 1
[0041] Example
Embodiment 7
[0042] Example 7 Preparation of 100mg / ml Fulvestrant Injection
[0043] It is the same as Example 1, but during the preparation process, "filter 1-2 times through a 0.2 micron filter" is changed to "sterilize at a high temperature of 121° C. for 12 minutes".
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com