Biodegradable high-polymer bonded photoactive Pt (IV) anticancer medicament micelle and preparation method thereof
A biodegradable and macromolecular technology, applied in the field of chemical synthesis of new drugs and their preparations, can solve problems such as lack of photoactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
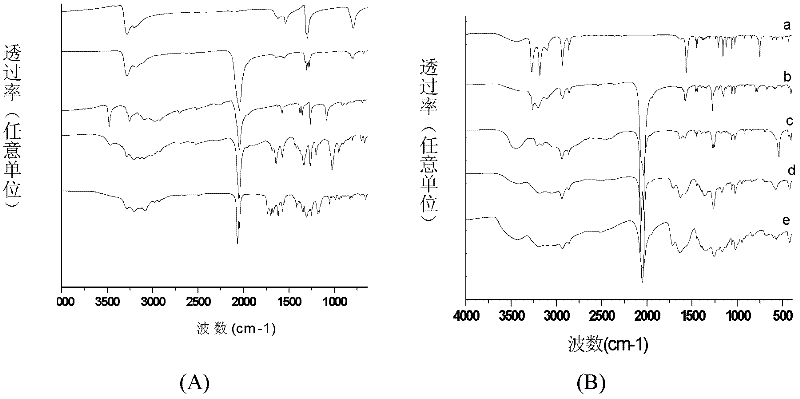
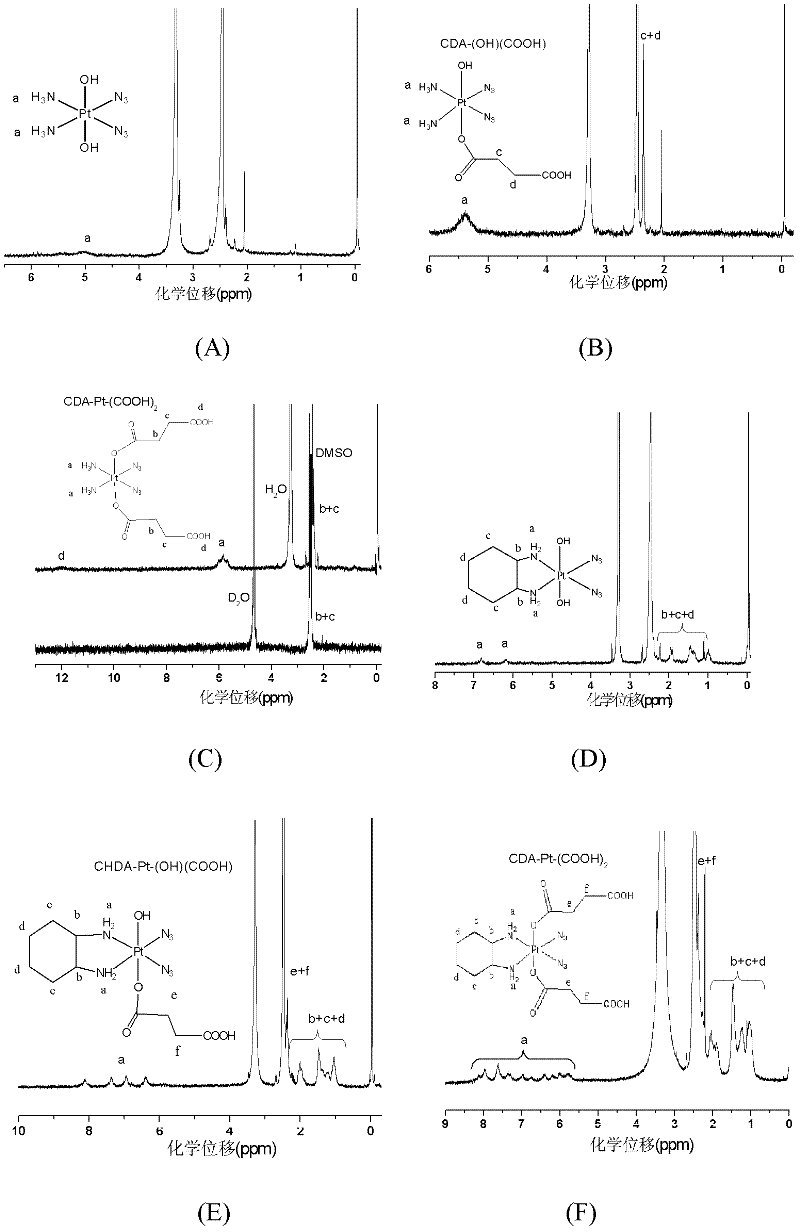
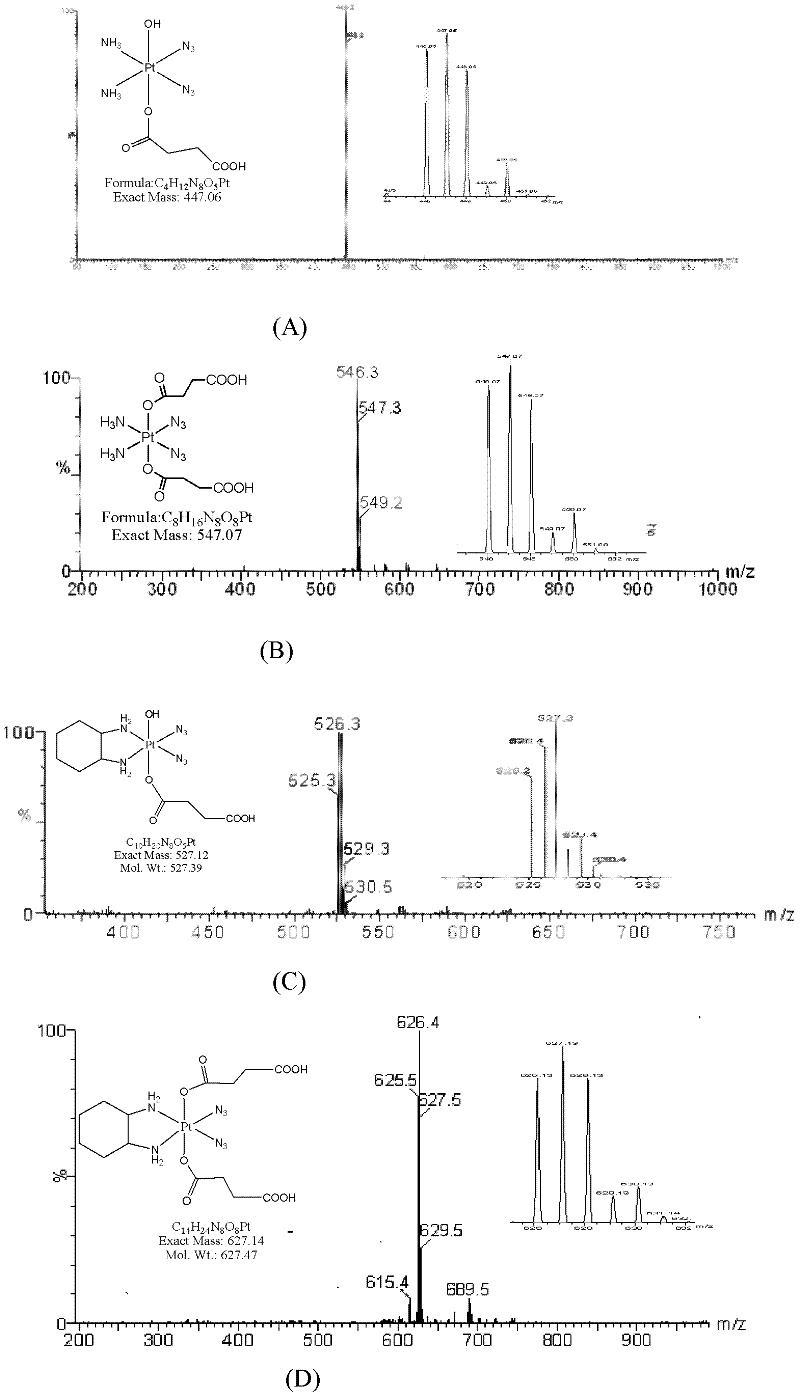
[0078] The present invention also provides a method for preparing a biodegradable polymer-bonded photoactive Pt(IV) anticancer drug, the preparation of the anticancer drug shown in formula 1 includes the following steps:
[0079] (1) Axial dihydroxy photoactive tetravalent platinum complex Pt(IV)-(OH) 2 React with an acid anhydride, introduce a ligand with a carboxylic acid in the axial direction of tetravalent platinum, and obtain a photoactive Pt(IV) complex with a carboxyl group in the axial direction. The reaction products are collectively referred to as "photoactive Pt(IV) with a carboxyl group". as "Pt(IV)-(OH) 2-x (COOH) x , with the following structure:
[0080]
[0081] In the formula, 1≤x≤2, wherein R is the part of the acid anhydride molecule that removes the carboxyl group, which can be an alkane, cycloalkane or aromatic structure, and the acid anhydride is succinic anhydride, glutaric anhydride, adipic anhydride, phthalic anhydride One of formic anhydride, c...
Embodiment 1
[0122] Example 1: Axial dihydroxy photoactive tetravalent platinum complexes c, c, t-[Pt(IV)(CHDA)(N 3 ) 2 (OH) 2 ] preparation
[0123] (1) 1.14g (10mmol) trans-1,2-cyclohexanediamine and 4.15g (10mmmol) potassium chloroplatinite were dissolved in 100ml twice-distilled water, and stirred and reacted in the dark for 12 hours to obtain the complex of divalent platinum Substance c, c-[Pt(II)(CHDA)Cl 2 ]3.5g;
[0124] (2) 1.9 g (5 mmol) of c, c-[Pt(II)(CHDA)Cl obtained in the above step (1) 2 ] and the silver nitrate of 1.7g (10mmol) were dissolved in the distilled water of 100ml, at room temperature avoid light stirring reaction 12 hours, filter method removes precipitate AgCl, obtains divalent platinum hydrate nitrate c, c-[Pt 2+ (II)(CHDA)(H 2 O) 2 ](NO 3 - ) 2 ;
[0125] (3) c, c-[Pt obtained by the above step (2) 2+ (II)(CHDA)(H 2 O) 2 ](NO 3 - ) 2 The aqueous solution was reacted with 0.65g (10mmol) of sodium azide at room temperature for 4h to obtain the d...
Embodiment 2
[0128] Example 2: Photoactive platinum (IV) complex CDA-Pt(IV)-(OH) with carboxyl groups in the axial direction 2-x (COOH) x preparation of
[0129] 200mg (0.576mmol) CDA-Pt(IV)-(OH) 2 Dissolve in 16mL DMSO, add the succinic anhydride of the listed weight in Table 1, stir and react at room temperature for 12 hours to obtain a mixed solution, the mixed solution is precipitated with 200mL acetone, the precipitate is washed with acetone and ether successively, and vacuum-dried at room temperature to obtain CDA-Pt(IV)-(OH) 2-x (COOH) x , the results are shown in Table 1.
[0130] Table 1. CDA-Pt(IV)-(OH) 2 - Preparation of succinic anhydride complex
[0131]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com