Amphipathic oxaliplatin precursor, preparation method and application thereof
An oxaliplatin, amphiphilic technology, applied in the field of amphiphilic oxaliplatin precursors, can solve ototoxicity, neurotoxicity and bone marrow toxicity, reduction of the actual efficacy of oxaliplatin, gastrointestinal reactions, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1. Preparation of oxidized oxaliplatin
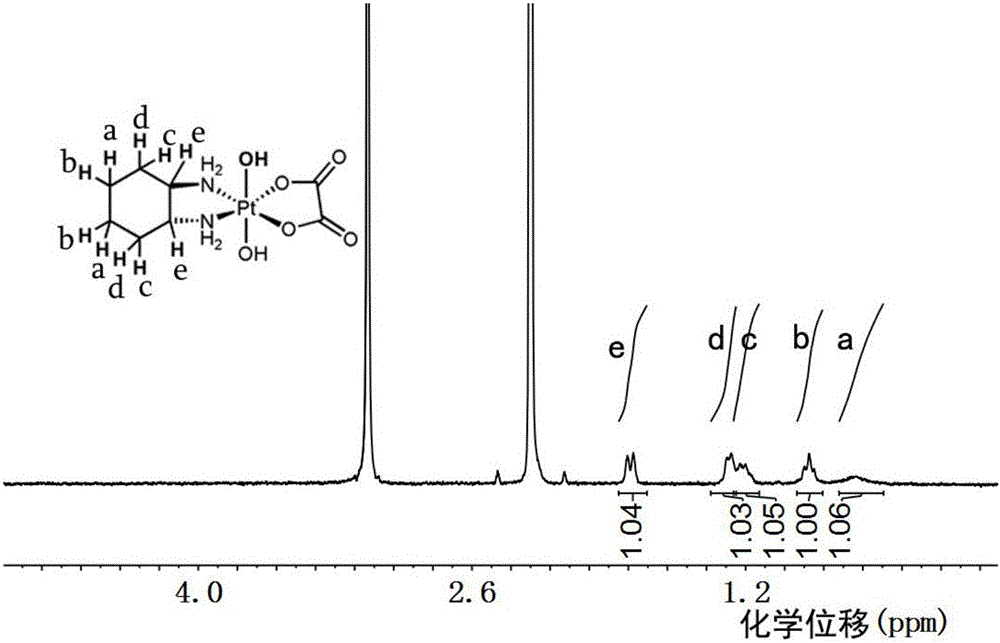
[0040] Weigh 500 mg of oxaliplatin, suspend it in 10 ml of deionized water, add 3 ml of 30% hydrogen peroxide, place it in a 50 ml round bottom flask, stir and react in the dark at 30 degrees Celsius for 12 hours, remove the solvent by rotary evaporation, add methanol to dissolve the resulting substance, Precipitate with ether and dry in vacuum to obtain oxaliplatin oxide. The resulting material was characterized by proton nuclear magnetic resonance spectroscopy, and the results were as follows: figure 1 shown.
Embodiment 2
[0041] Embodiment 2. Preparation of monocarboxylated oxaliplatin
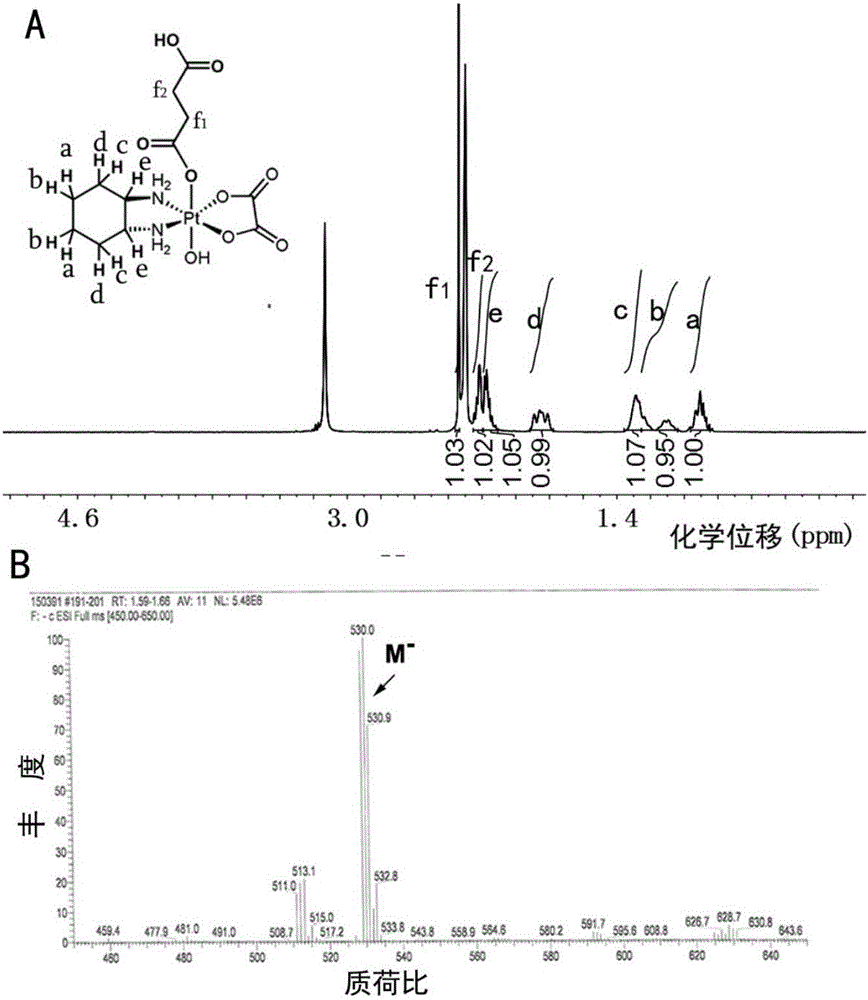
[0042] Take 215 mg of oxidized oxaliplatin prepared in Example 1, dissolve it in 5 ml of anhydrous dimethyl sulfoxide, add 50 mg of succinic anhydride, react at 25°C for 12 hours, precipitate with ether, dissolve the obtained substance in methanol, and then remove it by rotary evaporation Methanol, the resulting precipitate was washed with ether, and vacuum-dried to obtain monocarboxylated oxaliplatin. The resulting material was characterized by proton nuclear magnetic resonance and mass spectrometry, and the results were as follows: figure 2 shown.
Embodiment 3
[0043] Example 3. Alkylation of Monocarboxylated Oxaliplatin
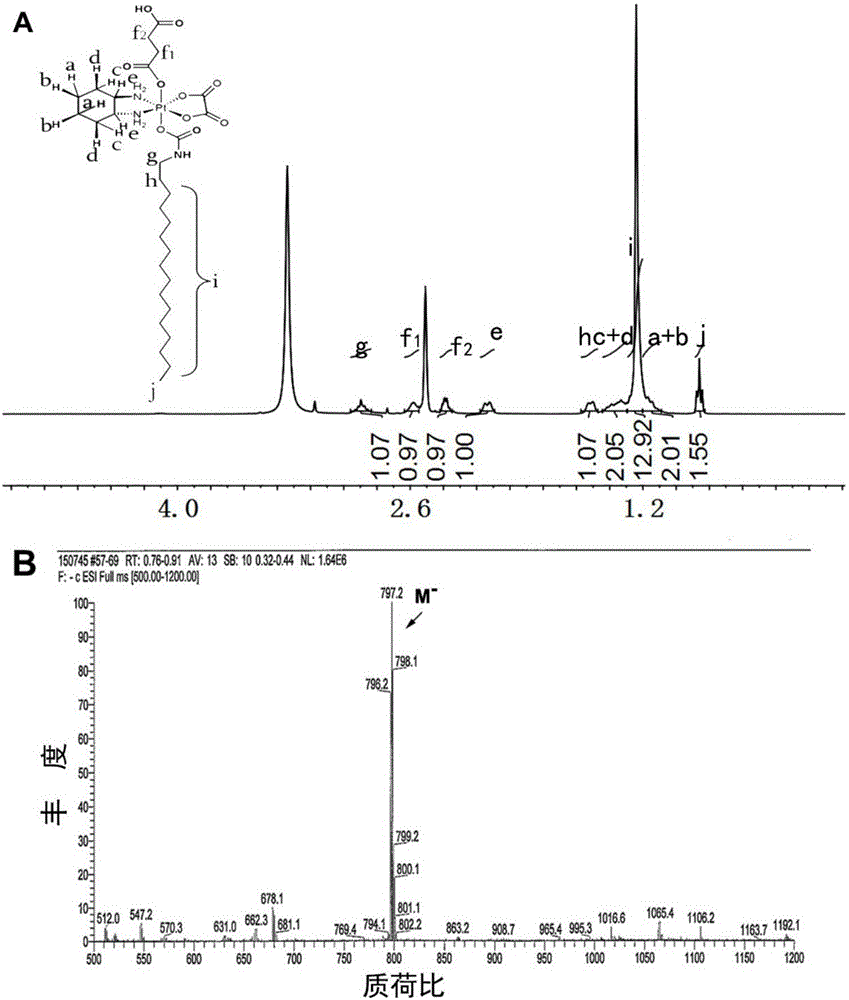
[0044] Take 60 mg of the monocarboxylated oxidized oxaliplatin prepared in Example 2, dissolve it in 3 ml of anhydrous N,N-dimethylformamide, add 45.33 mg of hexadecyl isocyanate, and place it at 25° C. to stir the reaction Concentrate overnight by rotary evaporation, wash the resulting substance with ether, and dry in vacuo to obtain monocarboxylated and alkylated oxaliplatin. The resulting material was characterized by proton nuclear magnetic resonance and mass spectrometry, and the results were as follows: image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com