Pegylated oxaliplatin prodrug as well as preparation method and application thereof
A technology of PEGylation and oxaliplatin, which is applied in the field of medicine and chemical industry, can solve the problems of limited clinical application, easy aggregation and leakage, and poor stability of liposome formulations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
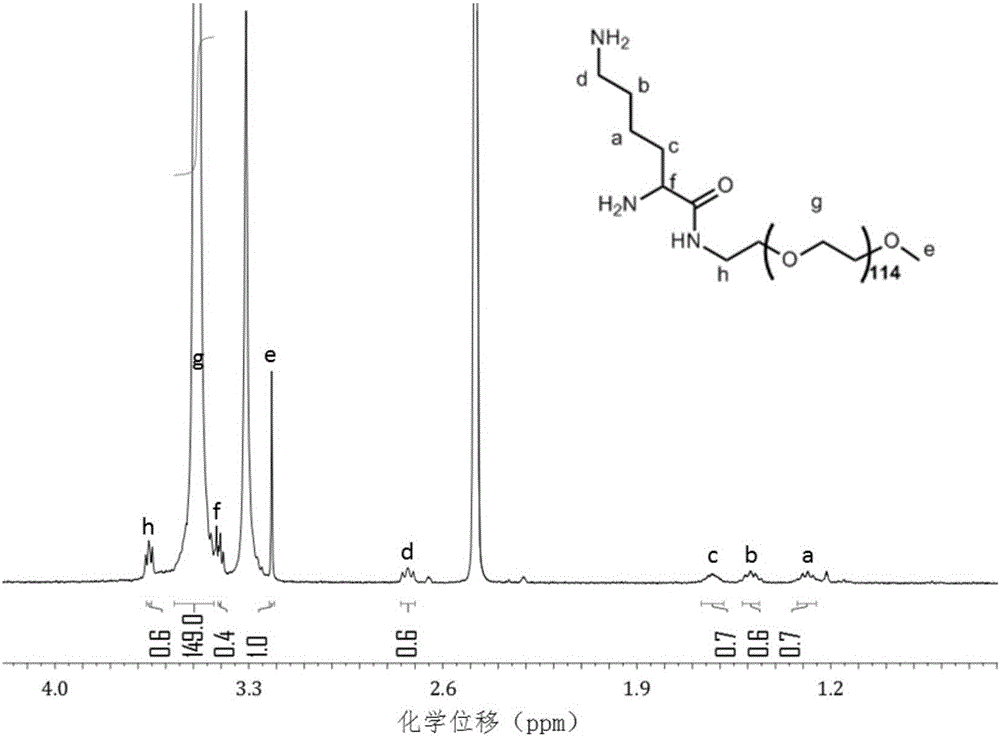
[0052] Embodiment 1: the preparation of lysinated polyethylene glycol
[0053] Take mPEG5k 1.0g, N2, N6-bis-tert-butoxycarboxy-L-lysine 277mg, carbodiimide hydrochloride 153mg, hydroxybenzotriazole 108mg, dissolve in 10ml of anhydrous acetonitrile, add 55 μl of triethylamine was stirred in the dark for 48 hours, and the organic solvent was spun dry, dissolved in 10 ml of water, transferred to a 3500 Da dialysis bag, dialyzed with deionized water for 48 hours, and the liquid in the bag was lyophilized.
[0054] Take 500mg of the above-mentioned product, add 5ml of dichloromethane to dissolve, slowly add 5ml of trifluoroacetic acid dropwise, avoid light and stir for 24 hours, spin to dry the organic solvent, add 10ml of water to dissolve, transfer to a 3500D dialysis bag, lyophilize after 48 hours of dialysis That is, lysinated polyethylene glycol. The resulting material was characterized by proton nuclear magnetic resonance spectroscopy, and the results were as follows: figur...
Embodiment 2
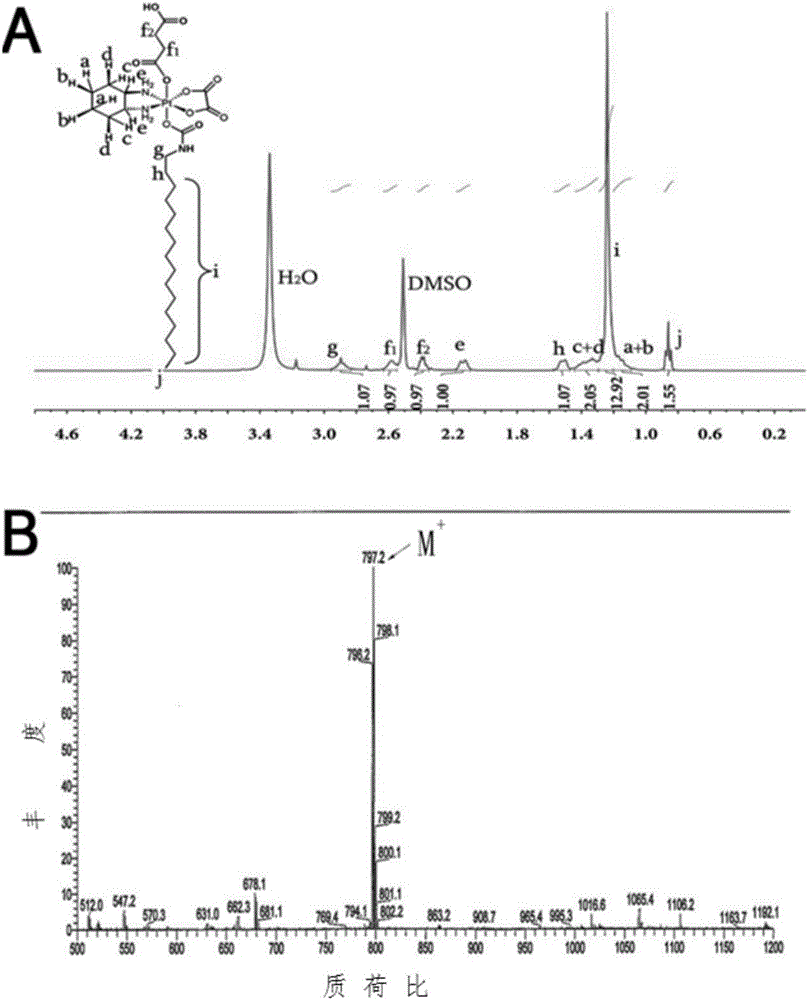
[0055] Embodiment 2: the preparation of single carboxylation and alkylation oxaliplatin
[0056] Weigh 500 mg of oxaliplatin, suspend it in 10 ml of deionized water, add 3 ml of 30% hydrogen peroxide, place it in a 50 ml round bottom flask, stir and react in the dark at 30 degrees Celsius for 12 hours, remove the solvent by rotary evaporation, add methanol to dissolve the resulting substance, Precipitate with ether and dry in vacuum to obtain oxaliplatin oxide.
[0057] Take 215 mg of oxidized oxaliplatin, dissolve it in 5 ml of anhydrous dimethyl sulfoxide, add 50 mg of succinic anhydride, react at 25 °C for 12 hours, and precipitate with ether, dissolve the obtained substance in methanol, and then remove the methanol by rotary evaporation. Washing and drying in vacuum to obtain monocarboxylated oxaliplatin.
[0058] Take 60 mg of monocarboxylated oxidized oxaliplatin, dissolve it in 3 ml of anhydrous N,N-dimethylformamide, add 45.33 mg of hexadecyl isocyanate, place it at 2...
Embodiment 3
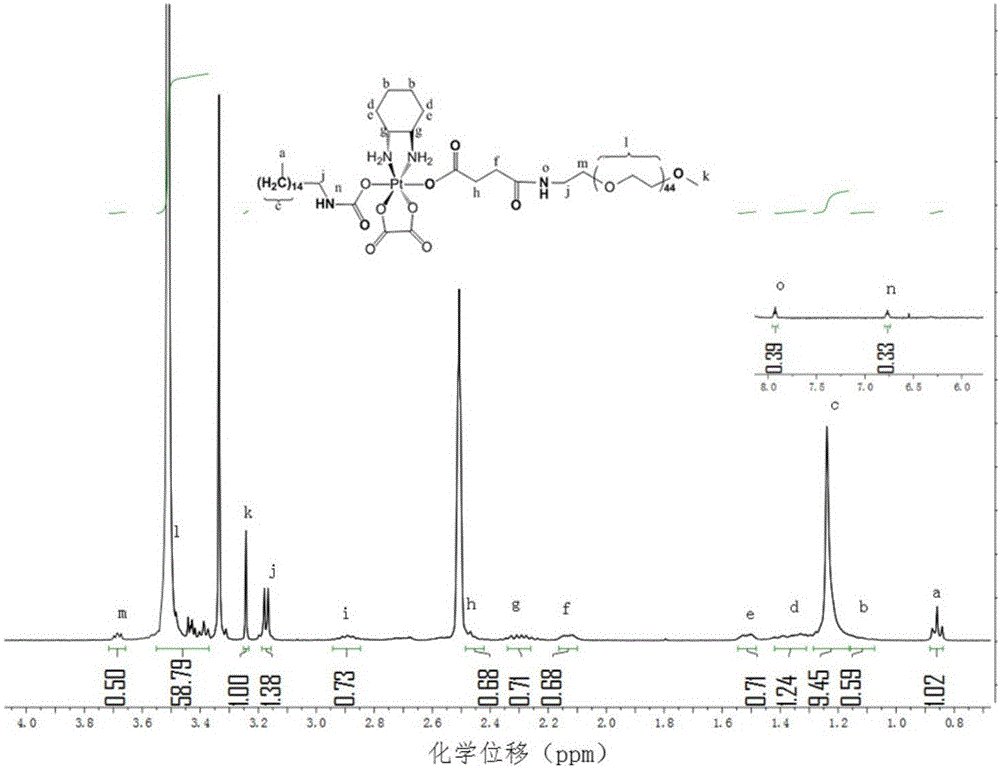
[0059] Embodiment 3: Preparation of pegylated oxaliplatin prodrug
[0060] 1. Preparation of PEGylated mono-oxaliplatin prodrug
[0061] Take the monocarboxylated and alkylated oxaliplatin 200mg prepared in Example 2, mPEG2k1.0g, carbodiimide hydrochloride 95.5mg, hydroxybenzotriazole 67.5mg, dissolve in 15ml of anhydrous acetonitrile , add 104 μl of triethylamine, stir for 48 hours in the dark, spin to dry the organic solvent, add 10ml of water to dissolve, select an ultrafiltration tube with a molecular weight cut-off of 30kDa to wash 5 times with water, and take the supernatant to freeze-dry to obtain PEGylation Oxaliplatin Prodrug. The resulting material was characterized by proton nuclear magnetic resonance spectroscopy, and the results were as follows: image 3 shown.
[0062] 2. Preparation of pegylated bisoxaliplatin prodrug
[0063] Take 500 mg of monocarboxylated and alkylated oxaliplatin prepared in Example 2, 1065.5 mg of lysinated polyethylene glycol in Exampl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com