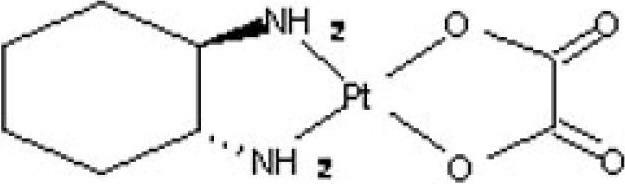
High optical purity trans-dextro oxaliplatin lyophilized powder injection and preparation method thereof
A freeze-dried powder injection, optical purity technology, applied in the field of medicine, can solve the problems affecting the safety of drug use, the levorotatory isomer has no drug activity and toxicity, and achieves the effects of overcoming risks, high optical purity, and good stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: The freeze-dried powder injection of trans-dextrooxaliplatin is made from the following components:
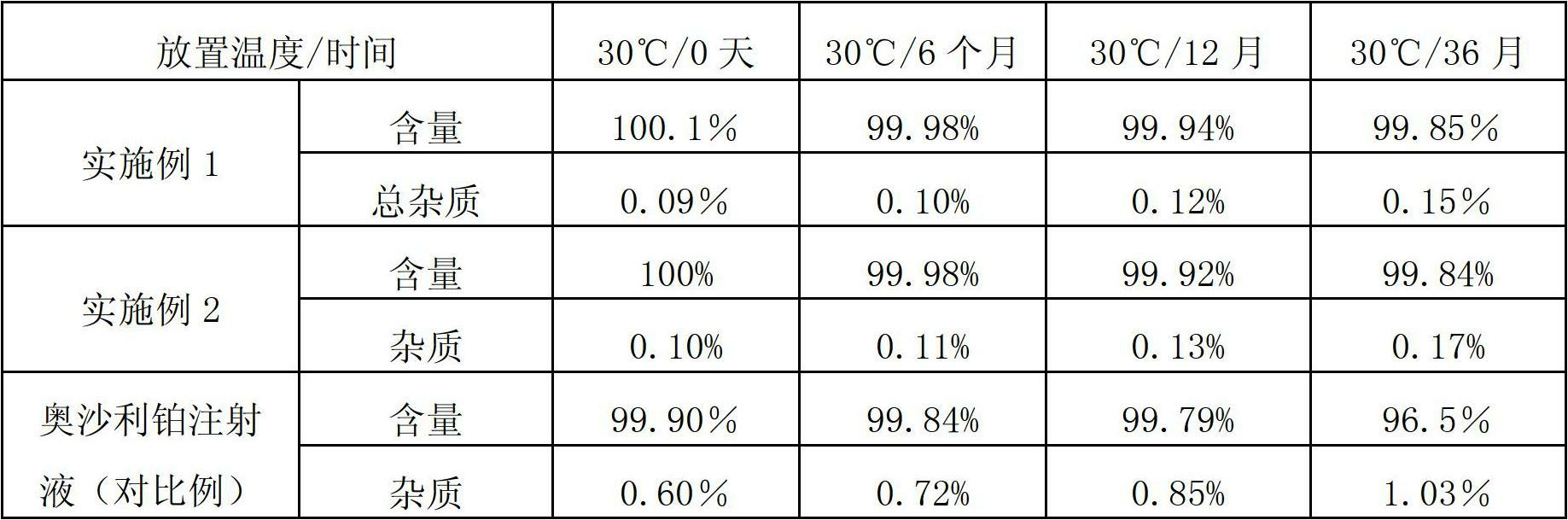
[0043] Trans-D-oxaliplatin 50mg, glycine 120mg, the optical purity of oxaliplatin is 100%.
[0044] Preparation:
[0045] (1) Dissolve 50mg of trans-D-oxaliplatin with an optical purity of 100% and 120mg of glycine in 10ml of water for injection, sterilize and filter, divide into 25mL glass bottles, and half add lyophilized rubber stoppers;
[0046](2) Put the packaged liquid medicine bottle on the shelf in the freeze-drying box for pre-freezing; first cool down the temperature of the plate layer to 5°C, keep it for 2 hours, and then quickly cool it down to -50°C within 60 minutes, After pre-freezing for 6 hours, carry out sublimation drying;
[0047] (3) When the vacuum degree in the drying oven reaches below 10Pa, the temperature of the shelf is slowly raised to 0°C in 120 minutes for sublimation drying, and the vacuum is controlled below 30Pa during t...
Embodiment 2
[0050] Embodiment 2: The freeze-dried powder injection of trans-dextro-oxaliplatin is made from the following components:
[0051] Trans-D-oxaliplatin 50mg, trehalose 135mg, the optical purity of trans-D-oxaliplatin is 99.99%.
[0052] Preparation:
[0053] (1) Dissolve 50mg of trans-D-oxaliplatin with an optical purity of 99.99% and 135mg of trehalose in 10ml of water for injection, sterilize and filter, divide into 25mL glass bottles, and half add lyophilized rubber stoppers ;
[0054] (2) Put the packaged liquid medicine bottle on the shelf in the freeze-drying box for pre-freezing: first cool down the temperature of the plate layer to 0°C, keep it for 2 hours, and then quickly cool it down to -60°C within 60 minutes , after pre-freezing for 4 hours, carry out sublimation drying;
[0055] (3) When the vacuum degree in the drying oven reaches below 30Pa, the temperature of the shelf is slowly raised to 0°C in 120 minutes for sublimation drying, and the vacuum is controlle...
experiment example 1
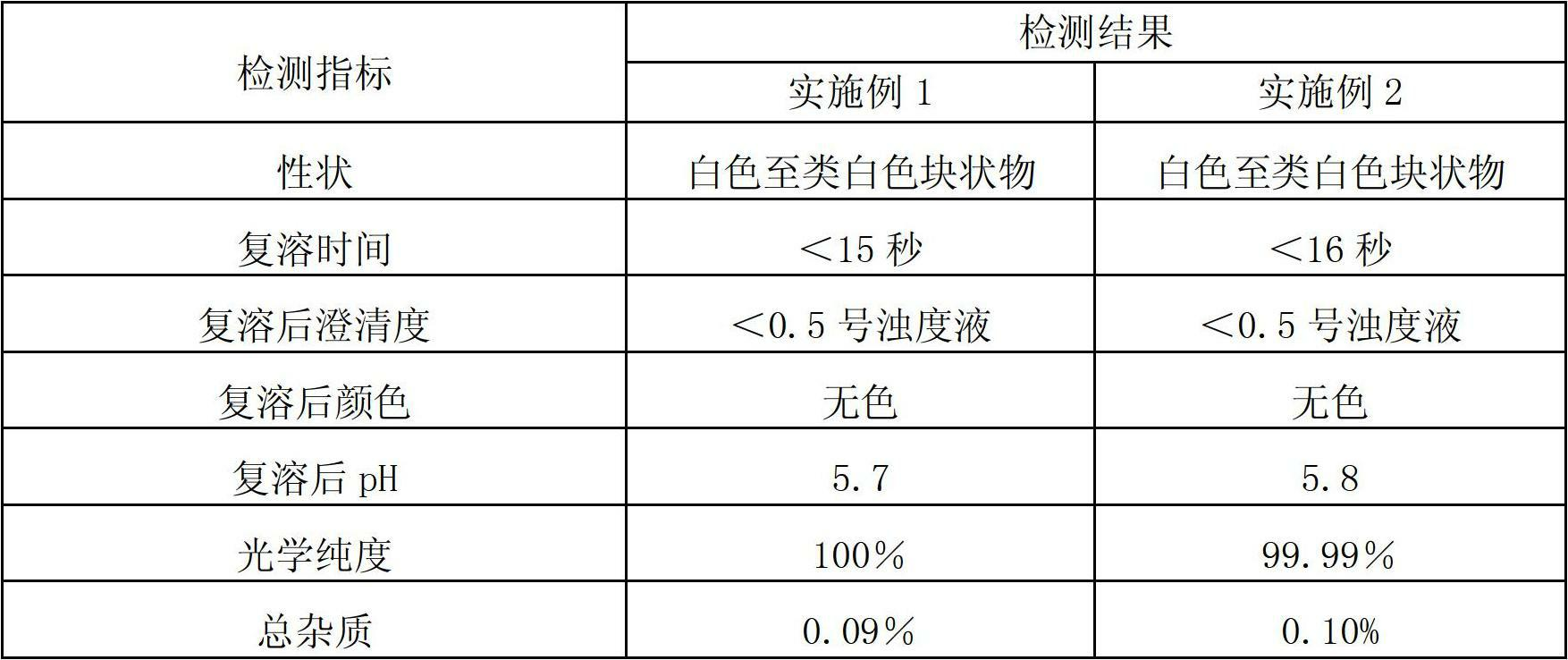
[0058] Experimental example 1, resolubility test
[0059] The trans-dex-oxaliplatin freeze-dried powder injection obtained in Examples 1 and 2 was detected, and the results are shown in Table 1 below:
[0060] Table 1. Reconstitution of lyophilized powder injection
[0061]
[0062] The above data show that the trans-D-oxaliplatin freeze-dried powder injection with high optical purity provided by the present invention has good reconstitution properties.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com