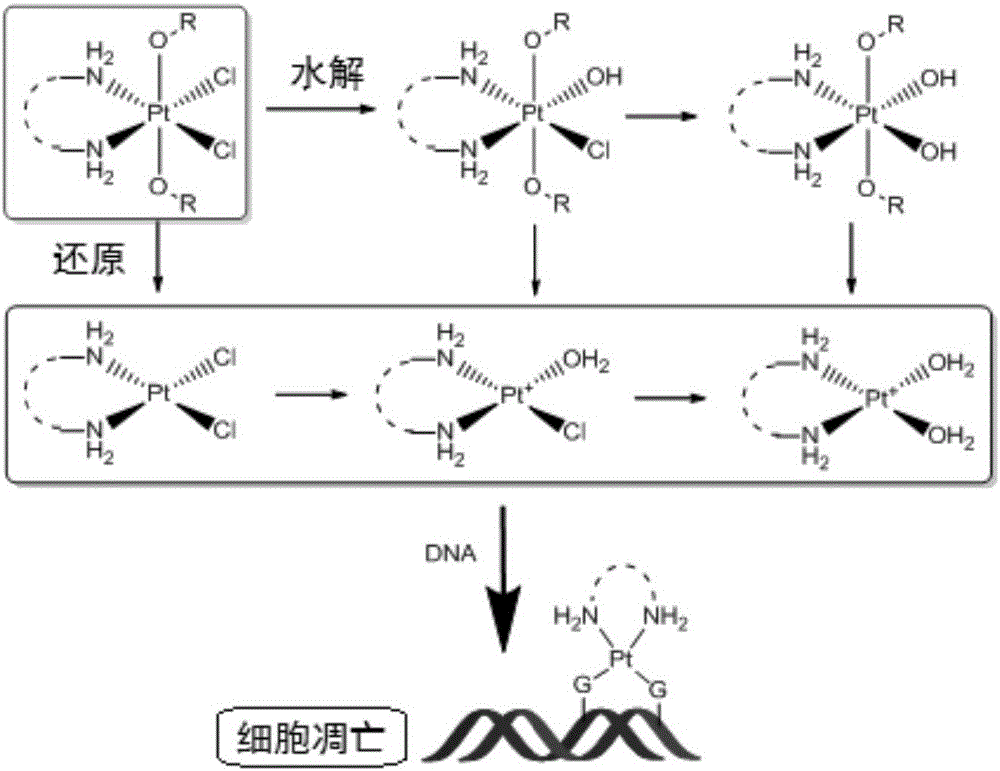
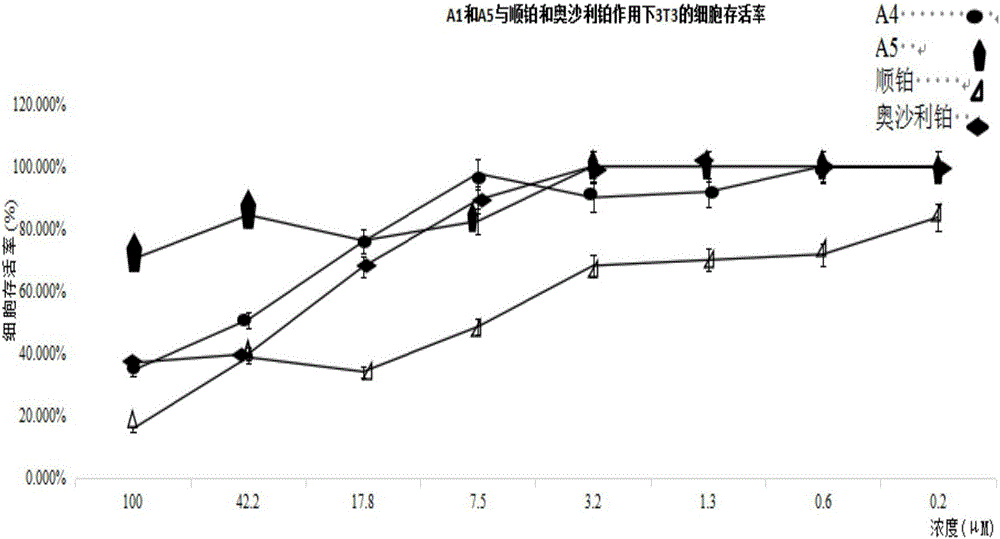
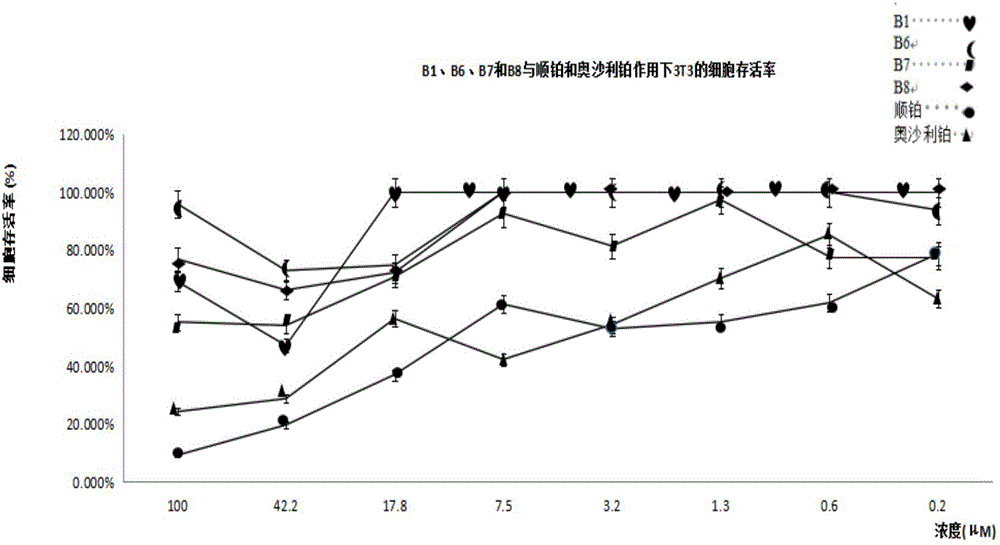
Quadrivalent-platinum glycosyl complex for treating tumors and preparation method thereof
A technology for tumor treatment and tetravalent platinum, which is applied in the preparation of sugar derivatives, medical preparations containing active ingredients, sugar derivatives, etc., can solve the problems of increased toxicity and side effects, inability to take oral medication, and cross-drug resistance, etc., to achieve Improve the water partition coefficient of ester, increase the maximum tolerated dose, and improve the effect of bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1: the synthesis of tetravalent cisplatin carboxylic acid
[0061]
[0062] C2HRMS:Calcd.forCl 2 h 8 N 2 o 2 Pt(M + ):332.96,found:332.9827.
[0063] C3HRMS:Calcd.forC 4 h 12 Cl 2 N 2 o 5 Pt(M + ):432.98,found:432.9847.
Embodiment 2
[0064] Example 2: Synthesis of Glycosylated Tetravalent Platinum Final Product A1
[0065]
[0066] The preparation method is as follows: C1 (divalent platinum compound cisplatin) is oxidized with hydrogen peroxide at 60-70°C and reacted for 4 hours to prepare C2 tetravalent cisplatin compound; C2 (1equiv) and succinic anhydride ( 4equiv) was dissolved in DMF, reacted overnight at 70°C under nitrogen protection, and then the oil pump was drained. After adding dichloromethane to precipitate a solid, C4 could be prepared by washing with chloroform, ether, etc.; the DMF solution of C4 was mixed with 2-(7 -Azobenzotriazole)-N,N,N',N'-Tetramethyluronium hexafluorophosphate DMF solution was mixed and stirred for 10 minutes, then added full acetylated glucosamine and N,N-di The DMF mixture of isopropylethylamine was reacted at room temperature in the dark for 24 hours to obtain A1.
[0067] A1: 1 HNMR (400MHz, CDCl 3 )δ6.41(d,J=146.8Hz,4H),5.47–4.79(m,14H),4.64–4.08(m,5H),4.02–...
Embodiment 3
[0068] Example 3: Synthesis of Glycosylated Tetravalent Platinum Final Product A2
[0069]
[0070] The preparation method is as follows: C1 (divalent platinum compound cisplatin) is oxidized with hydrogen peroxide at 60-70°C and reacted for 8 hours to prepare C2 tetravalent cisplatin compound; C2 (1equiv) and succinic anhydride ( 4equiv) was dissolved in DMF, reacted overnight at 70°C under nitrogen protection, and then the oil pump was drained. After adding dichloromethane to precipitate a solid, C4 could be prepared by washing with chloroform, ether, etc.; the DMF solution of C4 was mixed with 2-(7 -Azobenzotriazole)-N,N,N',N'-Tetramethyluronium hexafluorophosphate DMF solution was mixed and stirred for 20 minutes, then added full acetylated rhamnosamine and N,N - DMF mixed solution of diisopropylethylamine, A2 was obtained after reaction at room temperature in the dark for 48 hours.
[0071] A2: 1 HNMR (400MHz, CDCl 3 )δ5.28(s,6H),5.22–4.89(m,5H),4.72(s,1H),3.78(d,J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com