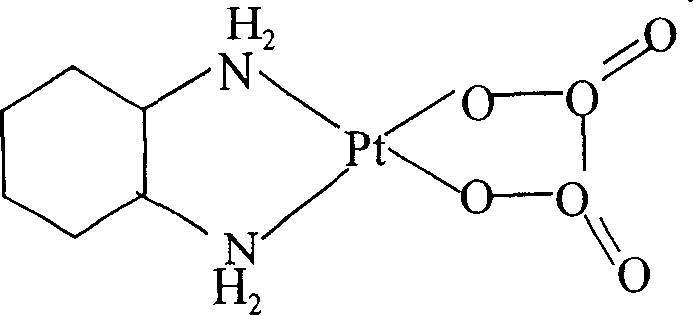
Liposome preparation containing Oxaliplatin
A technology of liposome preparation and oxaliplatin, which is applied in the directions of liposome delivery, active ingredients of heavy metal compounds, drug combination, etc., can solve problems such as stability of liposome preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1 liposome
[0037] This example provides a general strategy for preparing liposome compositions of the invention. Of course, other strategies that can be obtained by those skilled in the art by referring to this disclosed strategy can also be used. This example describes the use of reverse phase evaporation (REV) to prepare an embodiment of the liposomal formulations of the present invention (see, eg, US Patent No. 4,235,871 for a more detailed description).
[0038] In order to stably store the hydrophilic polymer in the lipid bilayer, the phospholipid derivative of the hydrophilic polymer can be prepared first, and then the phospholipid derivative, phospholipid and lipid can be used to prepare liposomes. Hydrophilic polymers are synthesized as derivatives in which phospholipid moieties are chemically attached to the polymer. The phospholipid portion of the derivative thus serves to stably hold the derivative in the lipid bilayer. Phos...
Embodiment 2
[0044] Example 2 Liposome Preparation
[0045] In this alternative mode of preparing liposomes, the lipid film forming step is carried out in a vessel partially filled with an inert solid contact material. Important changes can be in size, volume distribution, shape and composition of the exposed material. The primary characteristics of a contact substance are: (1) The contact substance is inert to the materials used in the formulation, in other words, there are no undesired gaps between the contact substance and the lipids, lipophilic substances, organic solvents or aqueous liquids used reaction, and (2) the contact substance has been solid during the reaction step, in other words, the contact substance should not dissolve or fragment, but should provide an appropriate solid surface to support the liquid phase film. Previous pilot tests using glass beads or glass spheres as the inert solid contact substance have proven that these materials are particularly suitable. It is a...
Embodiment 3
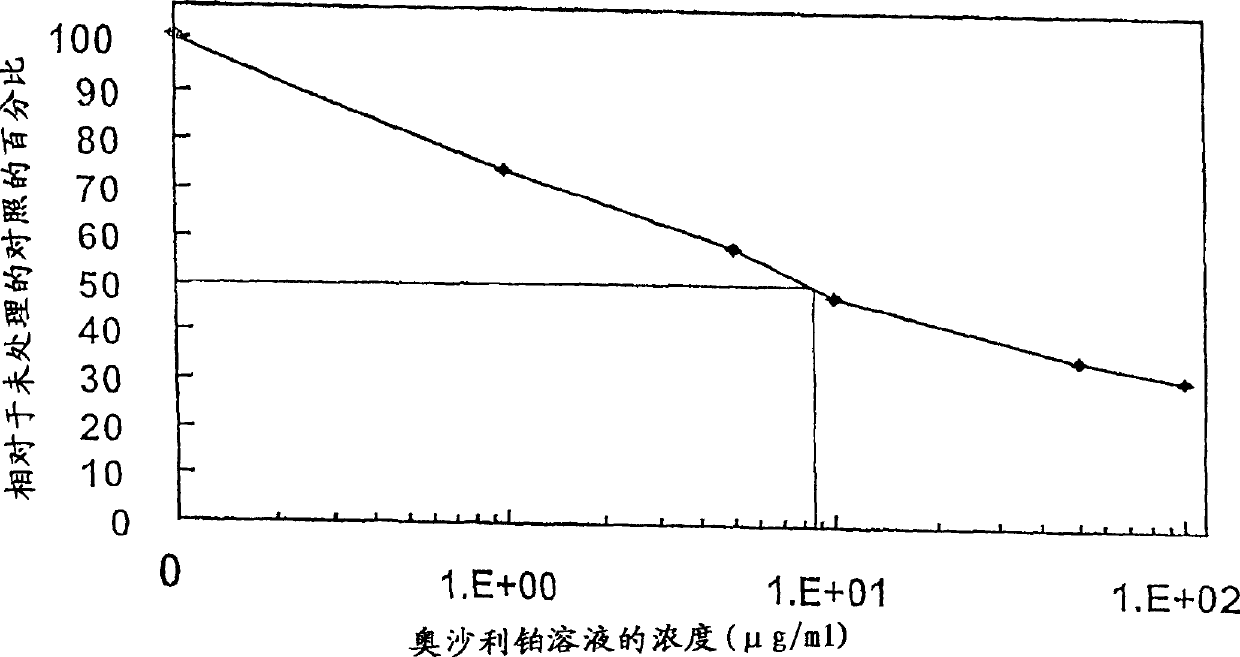
[0052] Example 3 Cytotoxicity of Oxaliplatin
[0053] An oxaliplatin solution was prepared by dissolving oxaliplatin in a 9% sucrose solution at a concentration of 8 mg oxaliplatin / ml solution.
[0054] AsPC-1 cells were cultured at 37°C in 5% CO in RPMI 11640 medium supplemented with 10% fetal bovine serum with various concentrations of oxaliplatin solutions. 2 Incubate for 4 hours. The medium was changed and the cells were cultured for an additional 48 hours. Assay cell viability with commercially available cytotoxicity assay kits, e.g., APO-ALERT TM Assay kit (Clontech, BD, Biosciences, Clontech, Palo. Alto, CA). Add culture medium to the cells and in 5% CO 2 Incubate for 2 hours, and measure the color development at 450nm (reference wavelength: 620nm). The result is shown in Figure 2. LD of oxaliplatin 50 >8ug / ml.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com