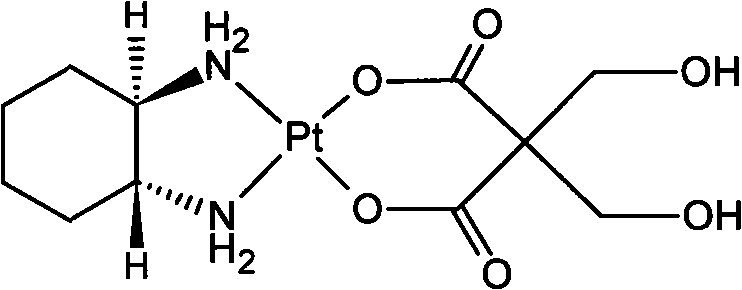
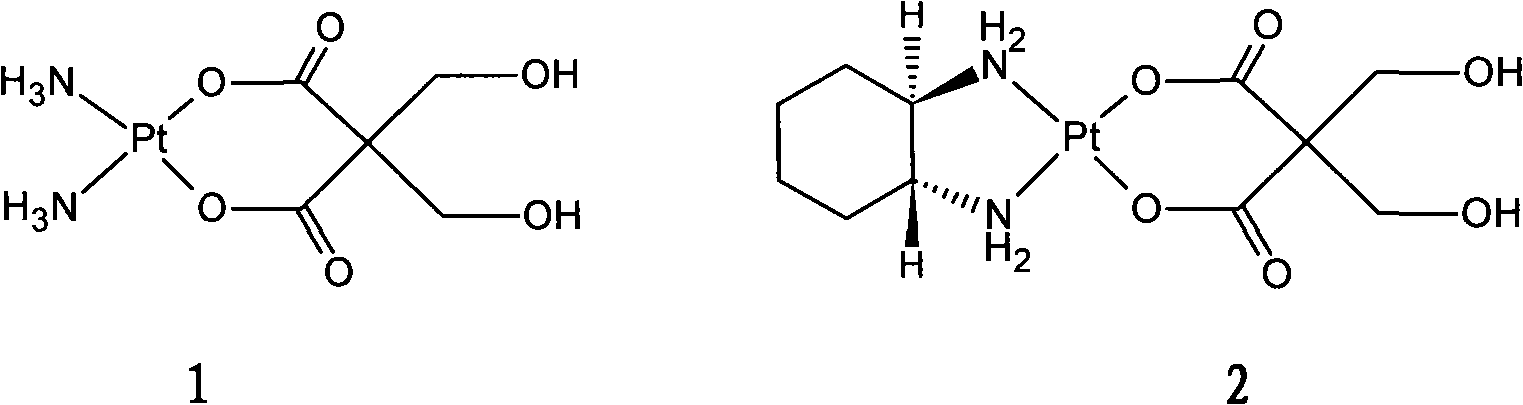
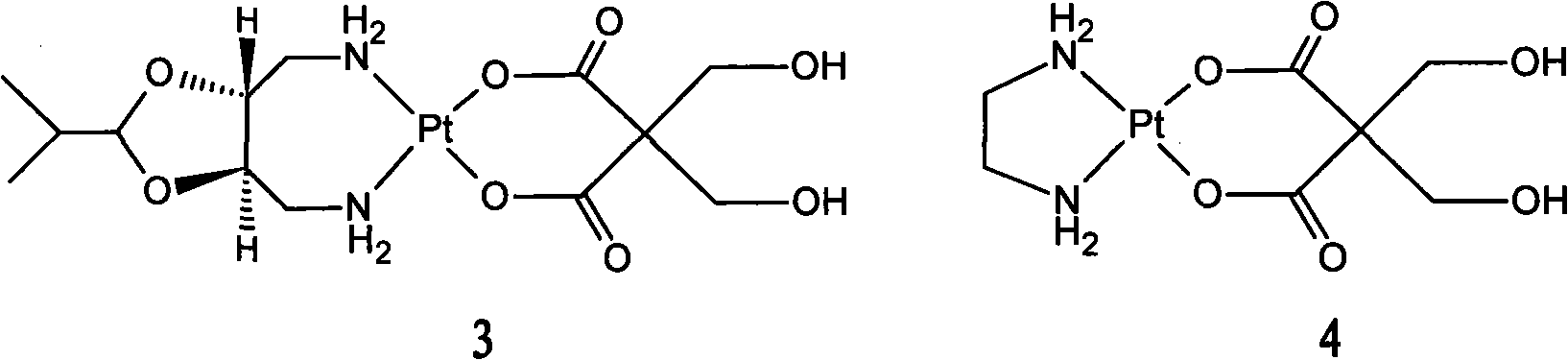
New oxaliplatin derivate
A technology for oxaliplatin and derivatives, applied in the field of oxaliplatin derivatives and their use as anticancer drugs, can solve the problems of high toxicity of oxaliplatin, and achieve the effect of good clinical application prospect and high water solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] (1) Preparation of 2,2-bis(hydroxymethyl)-1,3-silver malonate
[0018] Using commercially available diethyl 2,2-bis(hydroxymethyl)-1,3-malonate as raw material, obtain 2,2-bis(hydroxymethyl)-1,3-malonate after hydrolysis . Take 10g of 2,2-bis(hydroxymethyl)-1,3-malonic acid, dissolve it in 100ml of water, adjust the pH to 6-7 with 1mol / L NaOH, add 128mmol AgNO 3 (Excessive 5%) solution 100ml, produce white precipitate, filter and collect, after washing with water, ethanol, vacuum-dry at 60-70 ℃ for 4 hours, obtain 2,2-bis(hydroxymethyl)-1,3-malonic acid Silver 21g, yield 91%.
[0019] (2)cis-[Pt(II)A 2 I 2 ] Preparation of intermediate
[0020] Weigh 5g K 2 PtCl 4 (12mmol) was dissolved in 50ml of water, and the insolubles were removed by filtration. At 40°C, 50ml of an aqueous solution containing KI 12g (72mmol) was slowly added, and after reaction in the dark for 1 hour, an equimolar (12mmol) of 1R, 2R-ring Hexanediamine (A 2), a yellow precipitate was obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com