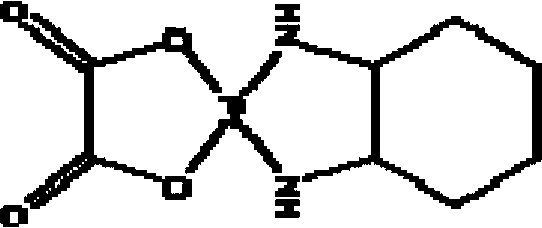
Oxaliplatin crystal compound and freeze-dried powder injection
An oxaliplatin and compound technology, which is applied in the field of oxaliplatin crystalline compounds and freeze-dried powder injections, can solve the problems of poor solubility and poor reconstitution performance of oxaliplatin, and achieves improved drug safety performance and improved dissolution. good performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]Take 100g of crude oxaliplatin, add a water:methanol solution with a volume ratio of 1300ml of crude oxaliplatin, and heat to reflux; after the crude oxaliplatin is dissolved, add 0.5 times of activated carbon for decolorization 30min, filter, add dropwise to the filtrate with a volume of oxaliplatin crude product weight 180ml and a volume ratio of 2.5:7.5 ether: isobutanol solution under stirring, the stirring is 23rmp, and the dropping is controlled dropping time 4 Add dropwise at a constant rate of about 1 minute; after dropping, stir and cool down, the stirring and cooling down is 10 minutes at 18rmp to 38°C, then 13rmp to 17°C for 15 minutes, let stand for 19 hours, filter, and use 6:4 ether : Washing with isobutanol solution and drying to obtain 97.8 g of the oxaliplatin crystalline compound, HPLC content 99.72%, mp 172.3-172.8°C.
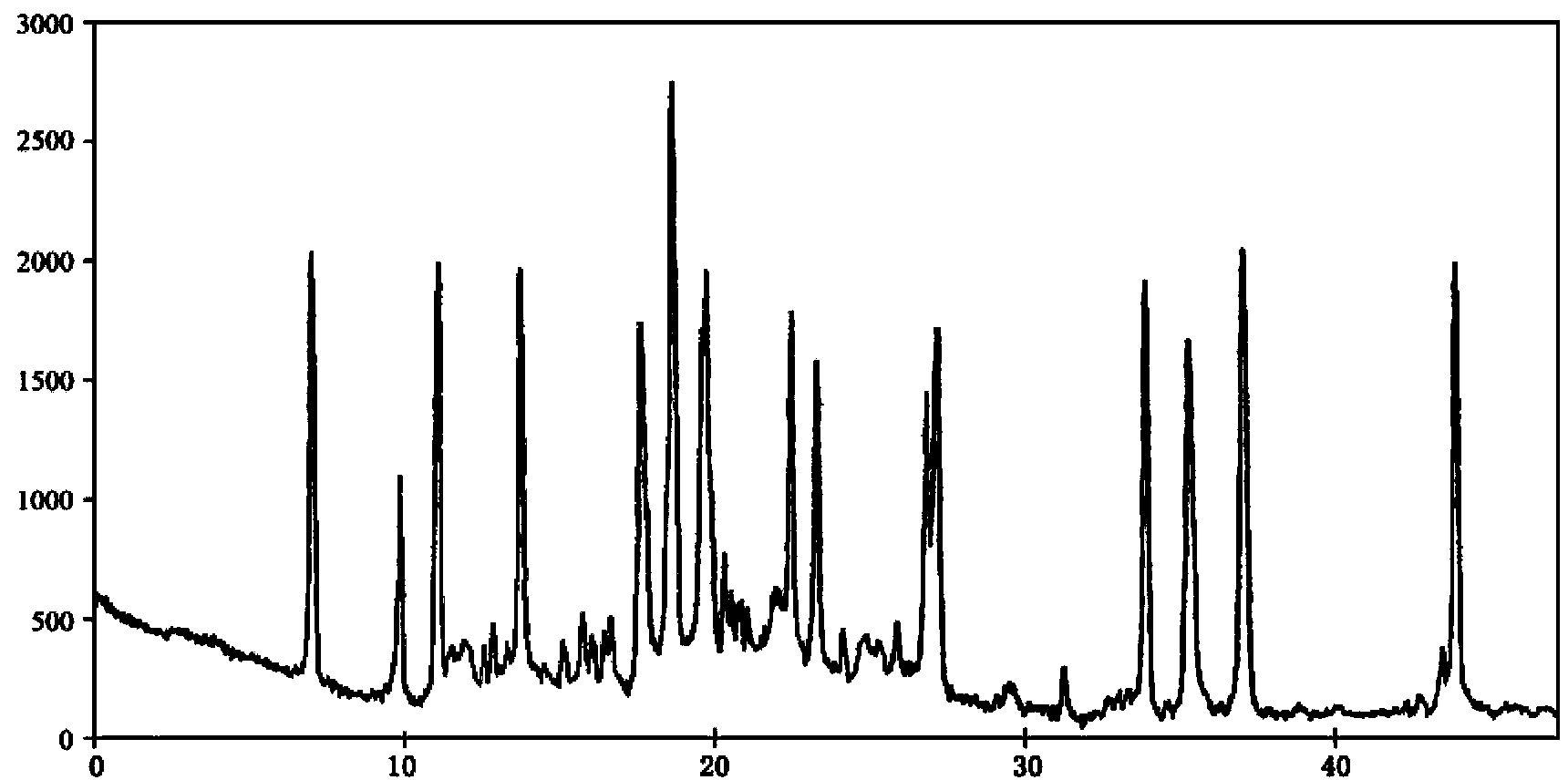
[0038] Measured by powder X-ray diffraction method, the X-ray powder diffraction pattern represented by 2θ±0.2° diffraction angle is a...
Embodiment 2
[0040] Take 100g of crude oxaliplatin, add a water:methanol solution with a volume ratio of 1200ml of crude oxaliplatin, and heat to reflux; after the crude oxaliplatin is dissolved, add 0.3 times of activated carbon to decolorize 30min, filter, add dropwise to the filtrate with a volume ratio of 2.5:7.5 ether: isobutanol solution with a volume ratio of 180ml of oxaliplatin crude product weight under stirring, the stirring is 20rmp, and the dropping is controlled dropping time 3.5 Add dropwise at a constant speed in about 1 minute; after dropping, stir and cool down, the stirring and cooling down is 10 minutes at 19rmp to 38°C, then 15min at 14rmp to 19°C, let stand for 18 hours, filter, and use 6:4 ether : Washing with isobutanol solution and drying to obtain 98.1 g of the oxaliplatin crystalline compound, HPLC content 99.51%, mp 172.2-173.0°C.
[0041] Measured by powder X-ray diffraction method, the X-ray powder diffraction pattern represented by 2θ±0.2° diffraction angle i...
Embodiment 3
[0043] Take 100g of crude oxaliplatin, add a water:methanol solution with a volume ratio of 1400ml of crude oxaliplatin, and heat to reflux; after the crude oxaliplatin is dissolved, add 0.2 times of activated carbon to decolorize 40min, filter, add dropwise to the filtrate under stirring, the volume ratio is 2.5:7.5 ether: isobutanol solution, the volume ratio of oxaliplatin crude product weight 160ml, described stirring is 20rmp, and described dropwise is to control dropping time 5 Add dropwise at a constant speed in about 1 minute; after dropping, stir and cool down. The stirring and cooling is to cool down to 37°C for 10 minutes under stirring at a rotating speed of 20rmp, and then cool down to 15°C for 15min under stirring at a rotating speed of 15rmp, let stand for 19 hours, filter, and use 6:4 ether : Washing with isobutanol solution and drying to obtain 95.2 g of the oxaliplatin crystalline compound, HPLC content 99.74%, mp 172.2-172.7°C.
[0044] Measured by powder X-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com