Enteral composition for the prevention and/or treatment of sepsis
A composition, sepsis technology, applied in the field of enteral composition, can solve problems such as undiscovered medium-chain triglycerides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
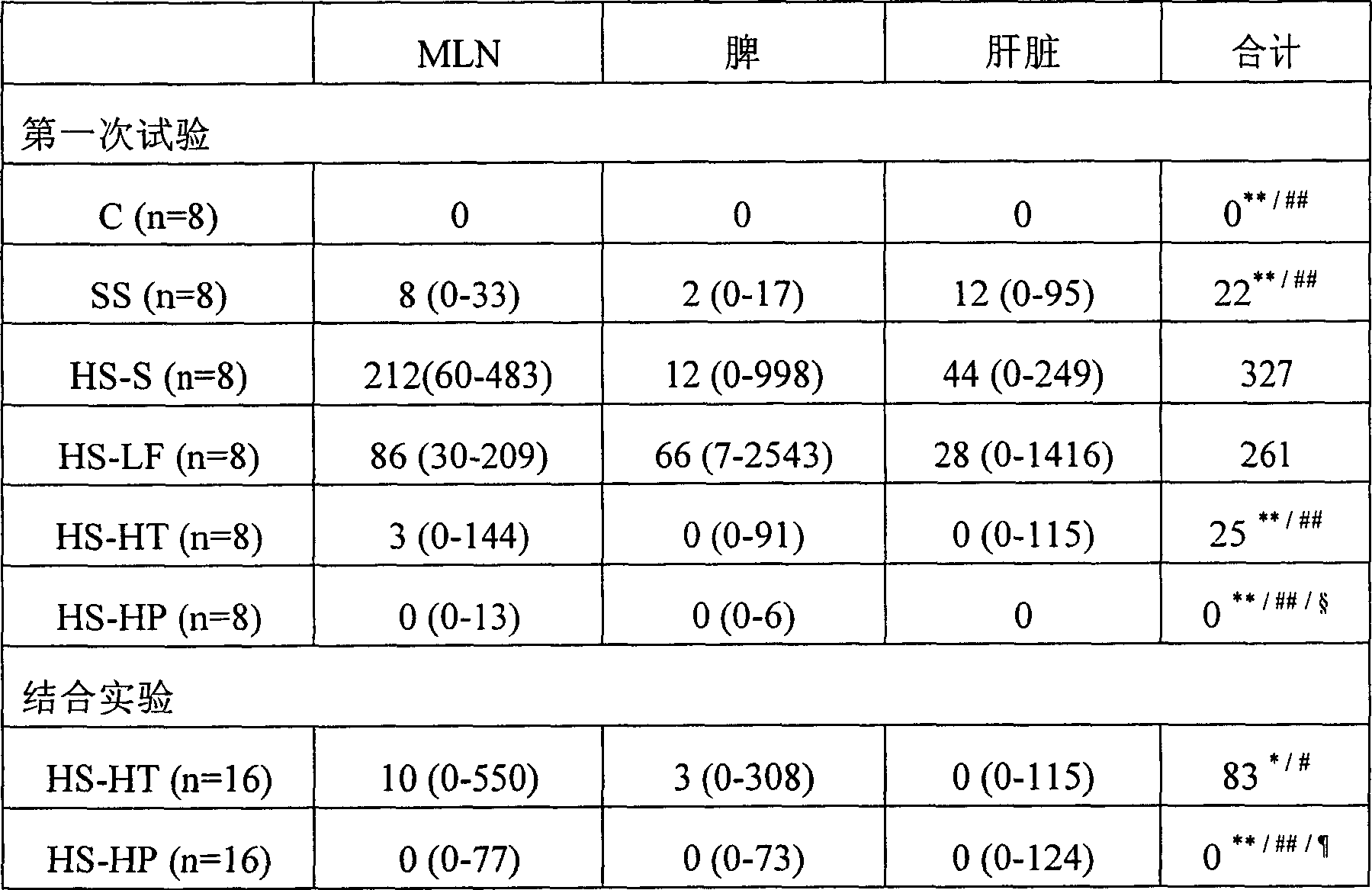
Image
Examples
Embodiment 1
[0094] Nutrient for tube feeding, which contains per 100ml:
[0095] Energy 125 kcal
[0096] Protein 1 7.5g
[0097] Sugar 1 14.5g
[0098] Lipids 4.2g includes:
[0099] Phospholipids 2.1g
[0100] Triglycerides 1 0.66g
[0101] Triglycerides 2 1.44g
Embodiment 2
[0104] Tube feeding nutrition which contains per 100ml:
[0105] Energy 100 kcal
[0106] Protein 2 4.0g
[0107] Sugar 2 12.3g
[0108] Lipids 3.9g. Contains:
[0109] Phospholipids 2.0g
[0110] Triglycerides 1 0.63g
[0111] Triglycerides 2 1.27g
[0112] Fiber 1g
Embodiment 3
[0114] Liquid nutrition as a supplement (suck-feed), which contains per 100ml:
[0115] Energy 100 kcal
[0116] Protein 2 4g
[0117] Sugar 3 8.8g
[0118] Lipids 5.4g includes:
[0119] Phospholipids 3.6g
[0120] Triglycerides 1 1.19g
[0121] Triglycerides 2 0.61g
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com