Preparation method of 6-bromoindole derivative
A technology of bromoindole derivatives and bromoindole, which is applied in the field of new preparation of pharmaceutical intermediates, and can solve problems such as synthesis difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
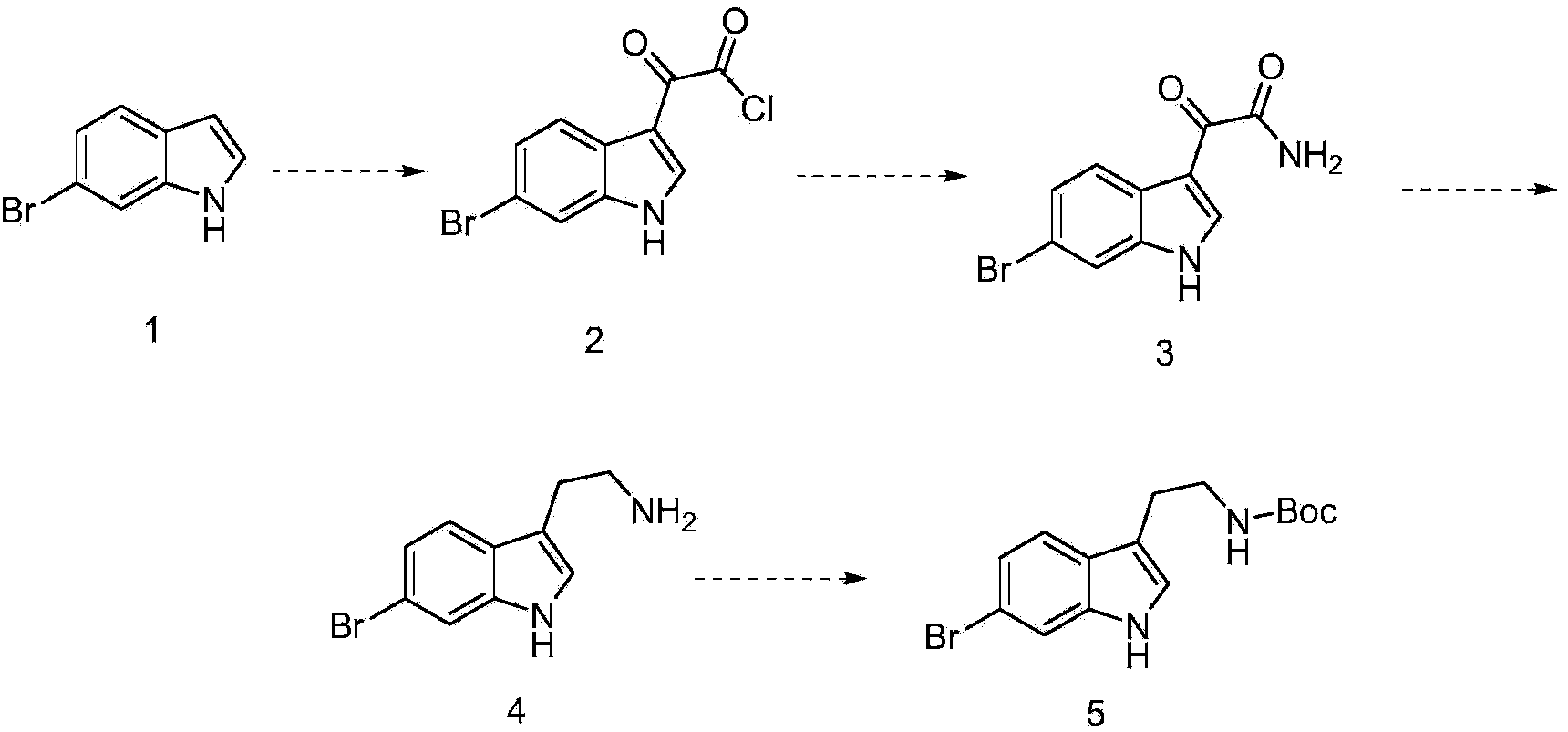
[0022] (1) Synthesis of 2-(6-bromo-1H-indol-3-yl)-2-oxoacetyl chloride
[0023] Add 40g of 6-bromoindole to 450ml of anhydrous dichloromethane, add 15g of aluminum trichloride, then add 60g of oxalyl chloride, heat to reflux for 2 hours, cool to room temperature, then add water, separate, dry, concentrate, The residue was subjected to column separation to obtain 47 g of 2-(6-bromo-1H-indol-3-yl)-2-oxoacetyl chloride.
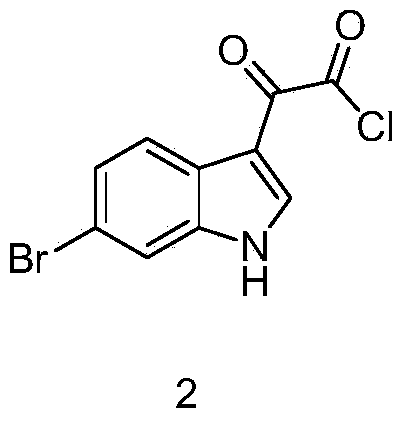
[0024] (2) Synthesis of 2-(6-bromo-1H-indol-3-yl)-2-oxoacetamide
[0025] Add 45g of 2-(6-bromo-1H-indol-3-yl)-2-oxoacetyl chloride to a mixture of 100ml of water and 500ml of ammonia water, stir at room temperature for 4 hours, add ethyl acetate for extraction, separate liquids and dry , concentrated, and the residue was separated on a silica gel column to obtain 32 g of 2-(6-bromo-1H-indol-3-yl)-2-oxoacetamide.
[0026] (3) Synthesis of 2-(6-bromo-1H-indol-3-yl)ethylamine
[0027] Add 30g of 2-(6-bromo-1H-indol-3-yl)-2-oxoacetamide into 200ml of anhydrous t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com