Enzyme-activity-improved ethanol dehydrogenase mutant and preparing method and application thereof
A technology of alcohol dehydrogenase and mutants, which is applied in the direction of oxidoreductase, fermentation, etc., can solve the problems of high price and low yield, and achieve the effect of reducing the amount of enzyme, reducing production cost and significant economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 Obtaining of wild-type alcohol dehydrogenase
[0029] Whole gene synthesis of the wild-type alcohol dehydrogenase gene shown in SEQ ID NO.2, and the following primers were designed according to the whole gene sequence:
[0030] PCR upstream primer: CTTTAAGAAGGAGATATACATATGAAAGCCGTCCAGTACACC
[0031] PCR downstream primer: GGCTTTGTTAGCAGCCGGATCTCATCAGGGAACCACCACGCC
[0032] Table 1 PCR reaction system
[0033] Reagent
Dosage / μl
PrimerStarPremix
25
PrimerFF
1
PrimerRR
1
DNA template
1
ddH2O
22
[0034] The PCR reaction conditions are as follows:
[0035] 98°C for 1min, 98°C for 10s, 55°C for 5s, 72°C for 5s / kbp, 30cycles, 16°C.
[0036] PCR amplification of wild-type alcohol dehydrogenase gene sequence;
[0037] Simultaneously perform PCR processing on the pET21a vector fragment:
[0038] PCR upstream primer: ATGTATATCTCTTCTTAAAG
[0039] PCR downstream primer: TGAGATCCGGCTGCTAACAAAG...
Embodiment 2
[0048] The acquisition of embodiment 2 alcohol dehydrogenase mutant
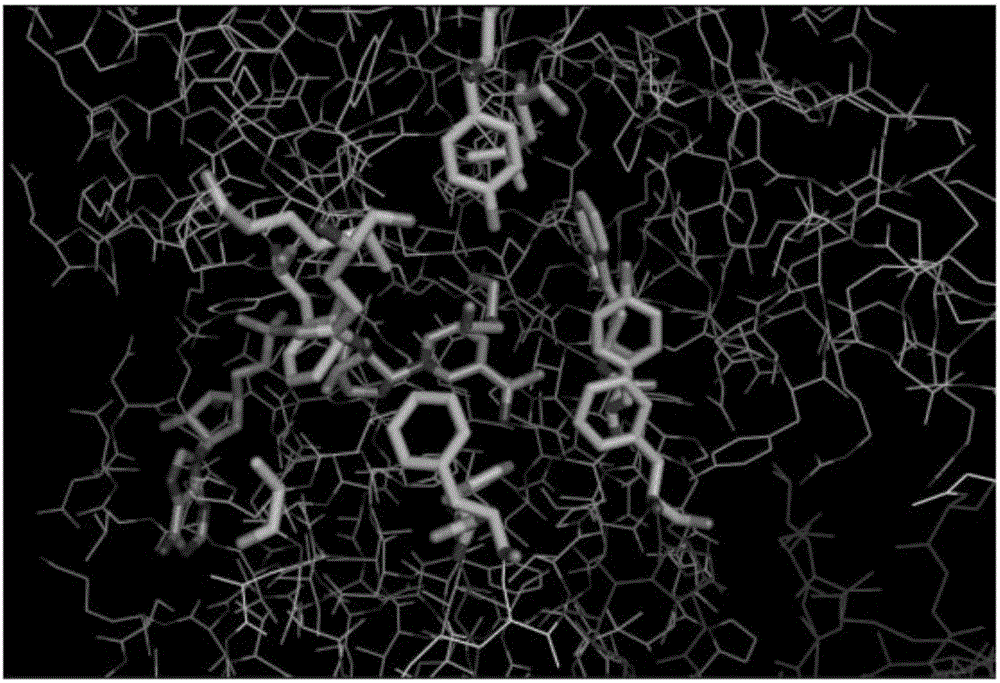
[0049] According to the three-dimensional structure of alcohol dehydrogenase (PDB: 2XAA), amino acids near the active center of alcohol dehydrogenase include F43, M47, Q51, A53, Y54, L119, A273, F281, F282, F286, Y294, W295. Since the amino acid changes near the active center may significantly affect the enzyme activity, the wild-type alcohol dehydrogenase was subjected to site-directed mutations by taking Q51, A53, Y54, A273, F286, and Y294 amino acid positions as examples. The primers listed in Table 2 were designed according to the mutation site.
[0050] Table 2 Site-directed mutagenesis primer sequences
[0051] Primer name
Primer sequence
KRED(ADH)_Q51M FF
CCGGCGGCGATGTACGCCTAC
KRED(ADH)_Q51M RR
GTAGGCGTACATCGCCGCCGG
KRED(ADH)_Q51L FF
CCGGCGGCGCTGTACGCCTAC
KRED(ADH)_Q51L RR
GTAGGCGTACAGCGCCGCCGG
KRED(ADH)_A53L FF
GCGCAGTACCCTCTACGGCCT...
Embodiment 3
[0080] The activity test of embodiment 3 alcohol dehydrogenase
[0081] The collected cells were resuspended to 3.5 g / L in 50 mM sodium phosphate buffer (pH 7.0), and homogenized twice using a high-pressure homogenizer and cell disruptor in an ice-water bath at 15,000 Psi to obtain a cell lysate.
[0082] The enzyme reaction activity system (5ml reaction system) includes: N-tert-butoxycarbonyl-piperidone 0.1g / ml, isopropanol 10% (v / v), Na 2 HPO 4 12H 2 O 24mg / ml, NaH 2 PO 4 2H 2 O 6.6mg / ml, MgCl 2 1mg / ml, coenzyme 0.4mg / ml, cell lysate 30% (v / v).
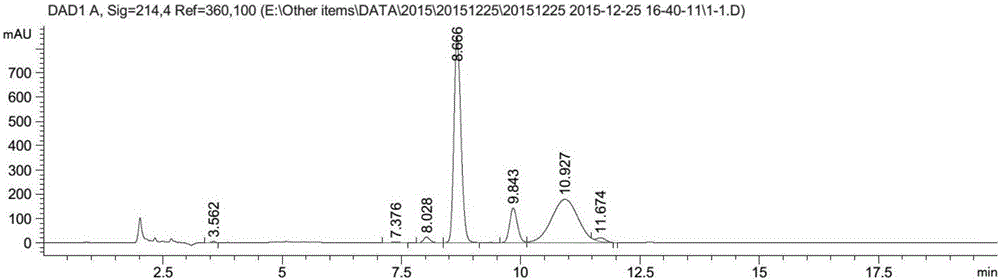
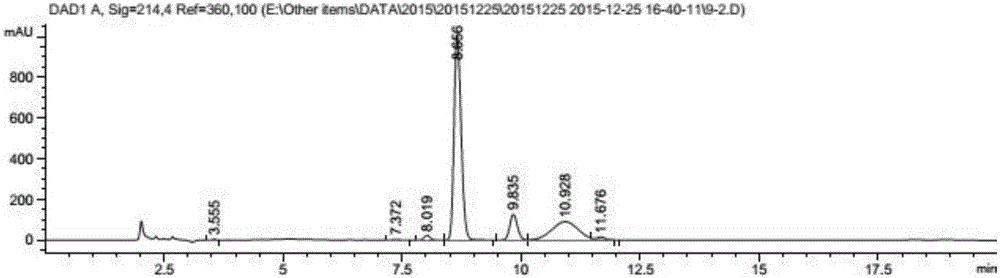
[0083] Place in a constant temperature shaker at 30°C and 200 rpm for reaction; after 15 hours of reaction, add 0.5ml of isopropanol; continue the reaction until the total reaction time is 22 hours. After the reaction, the sample was extracted by adding 2 times the volume of acetonitrile, centrifuged for 20 min to obtain the supernatant, filtered through a 0.45 μm filter membrane, detected by HPLC, and the conversion rate was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com