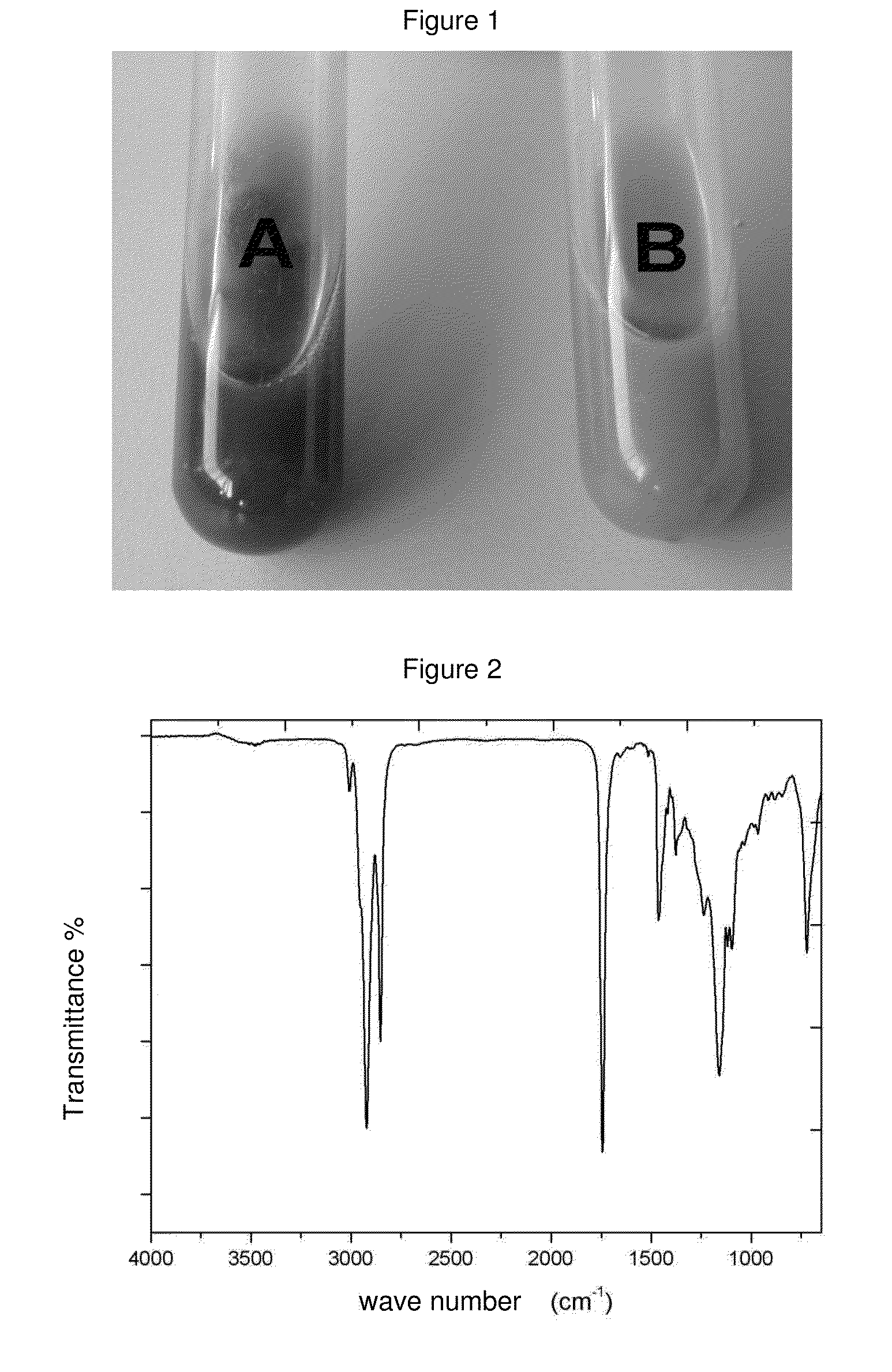
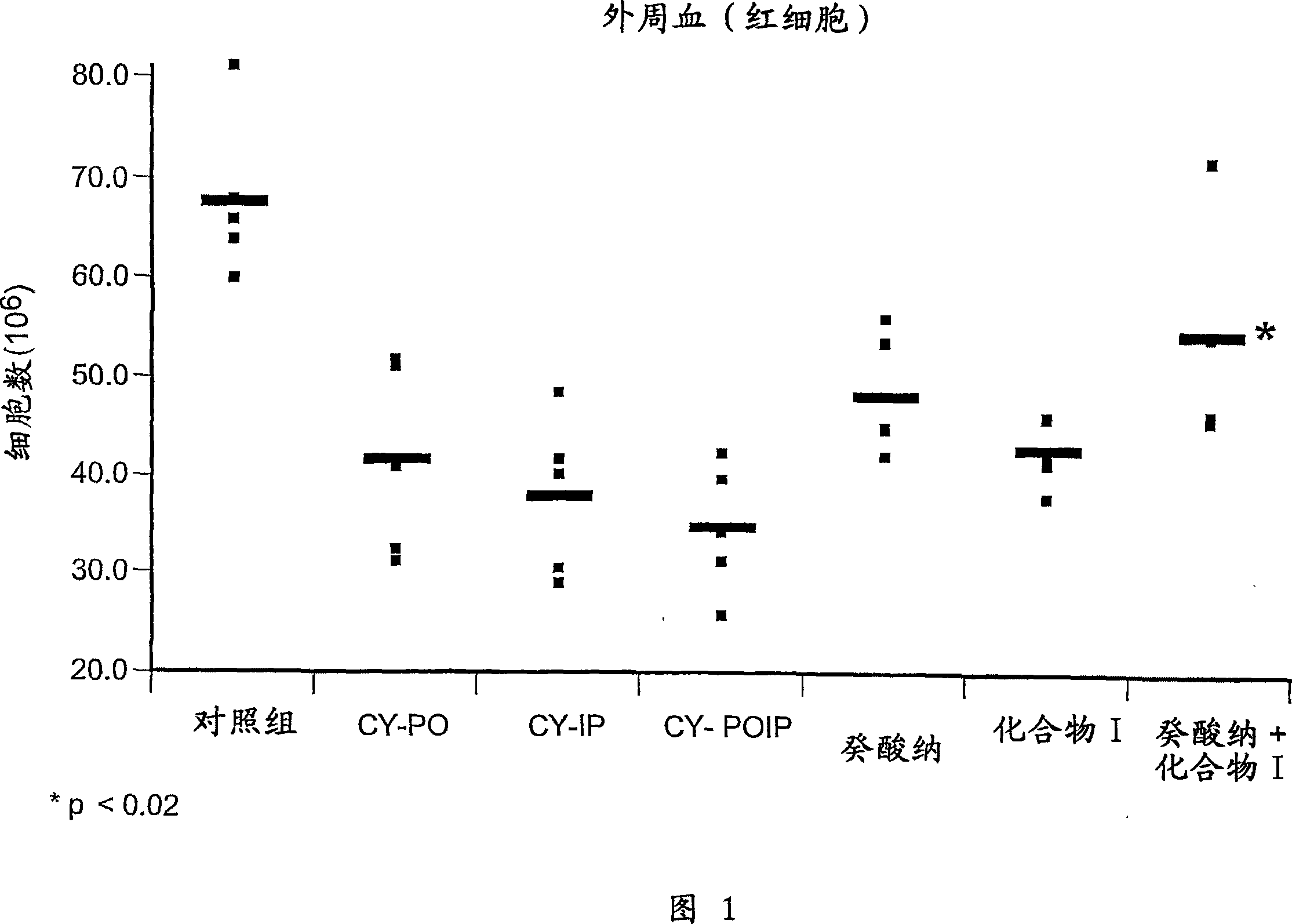
Patents
Literature
32 results about "Chemoprotective" patented technology
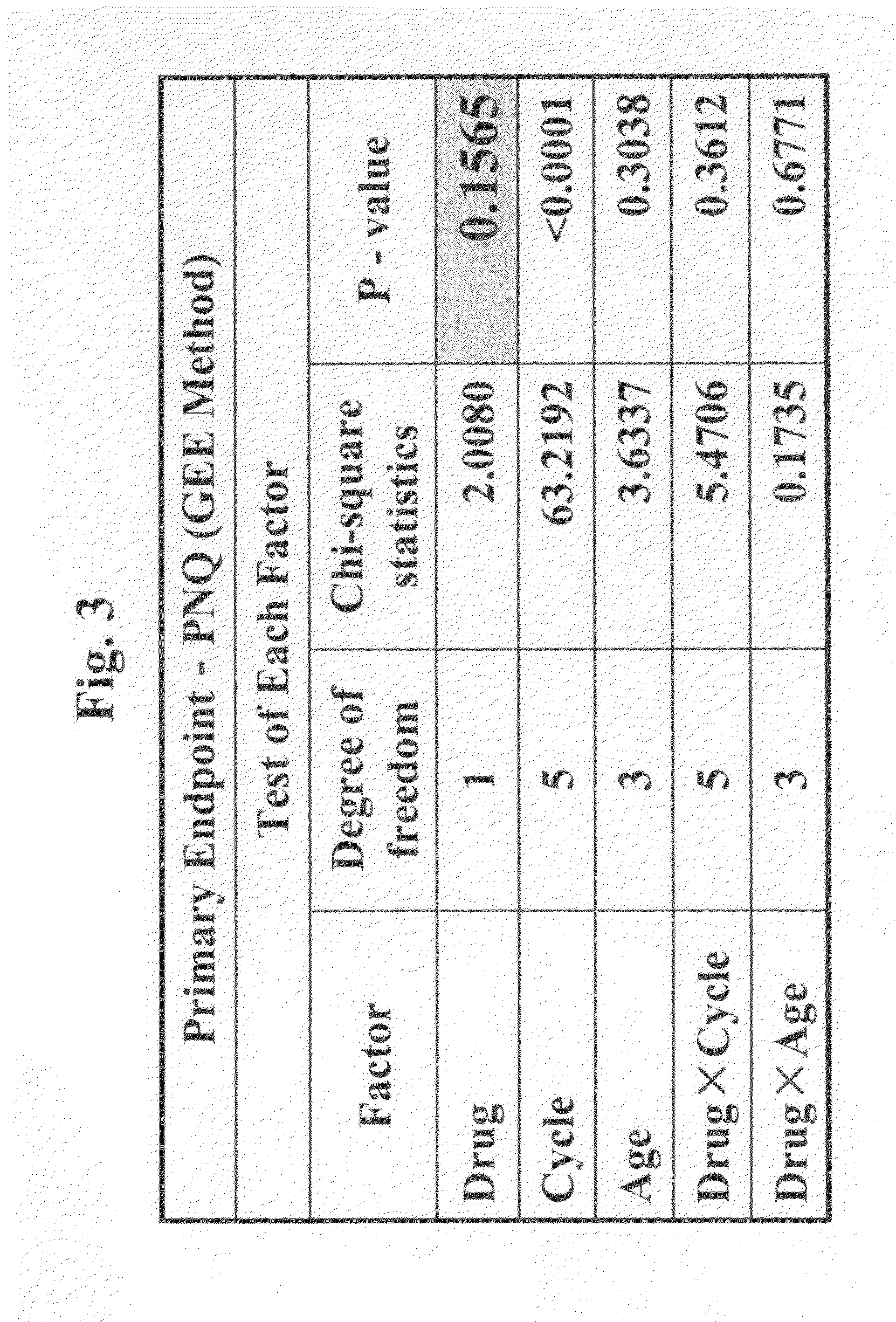
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A quality of some drugs used in cancer treatment. Chemoprotective agents protect healthy tissue from the toxic effects of anticancer drugs.
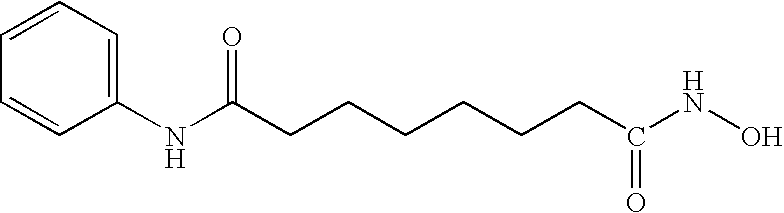
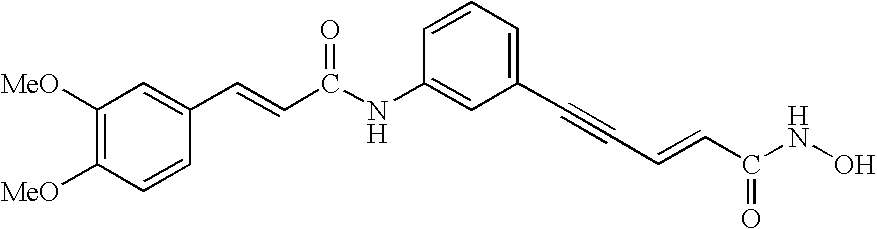
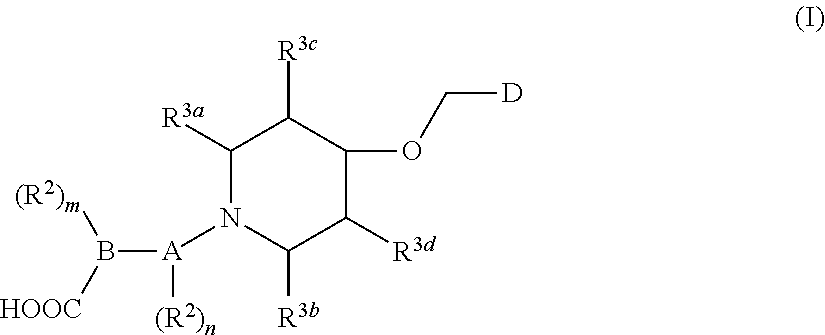
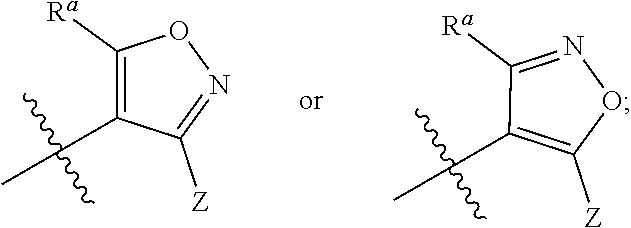
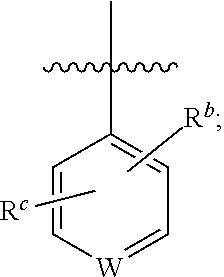
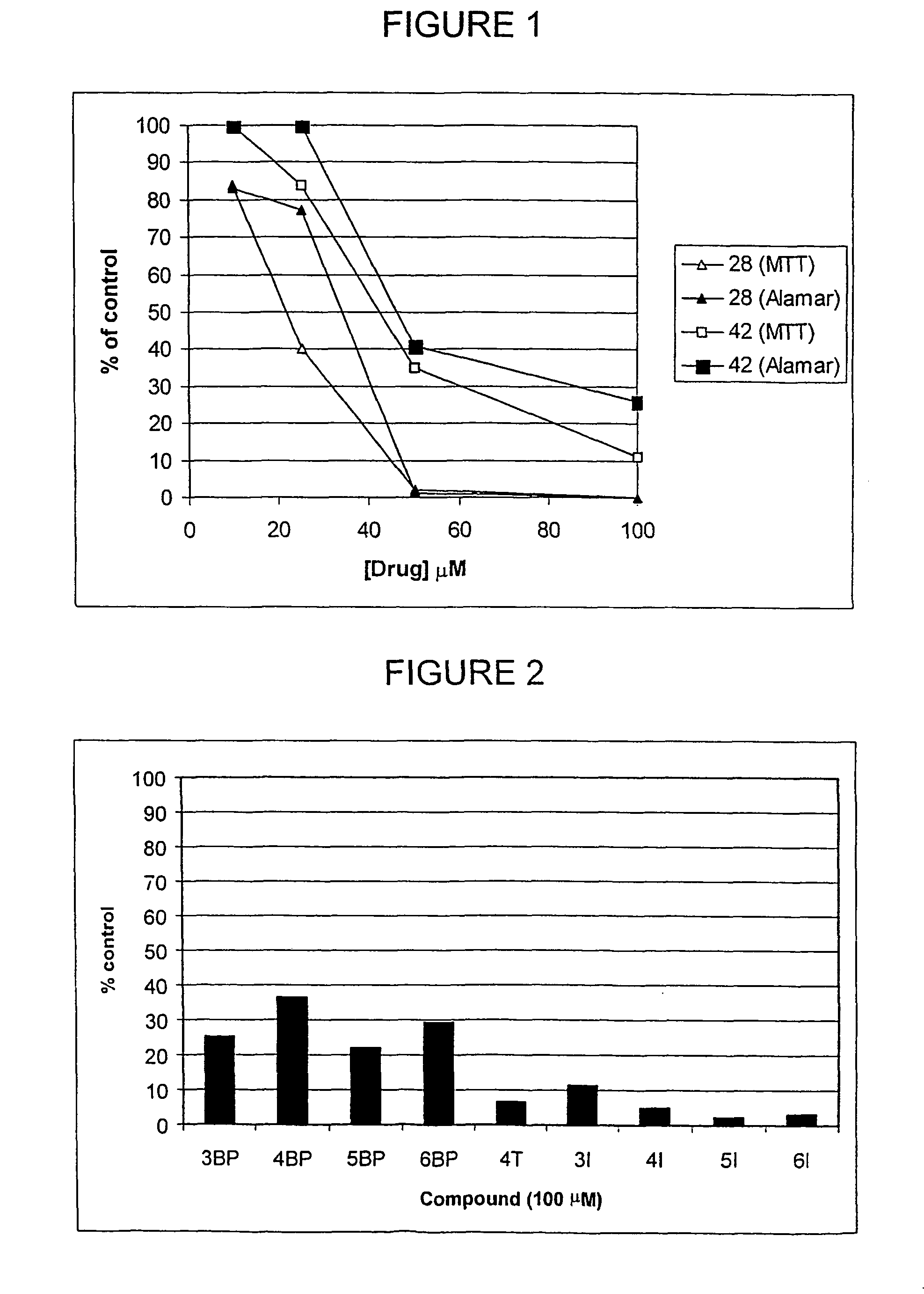
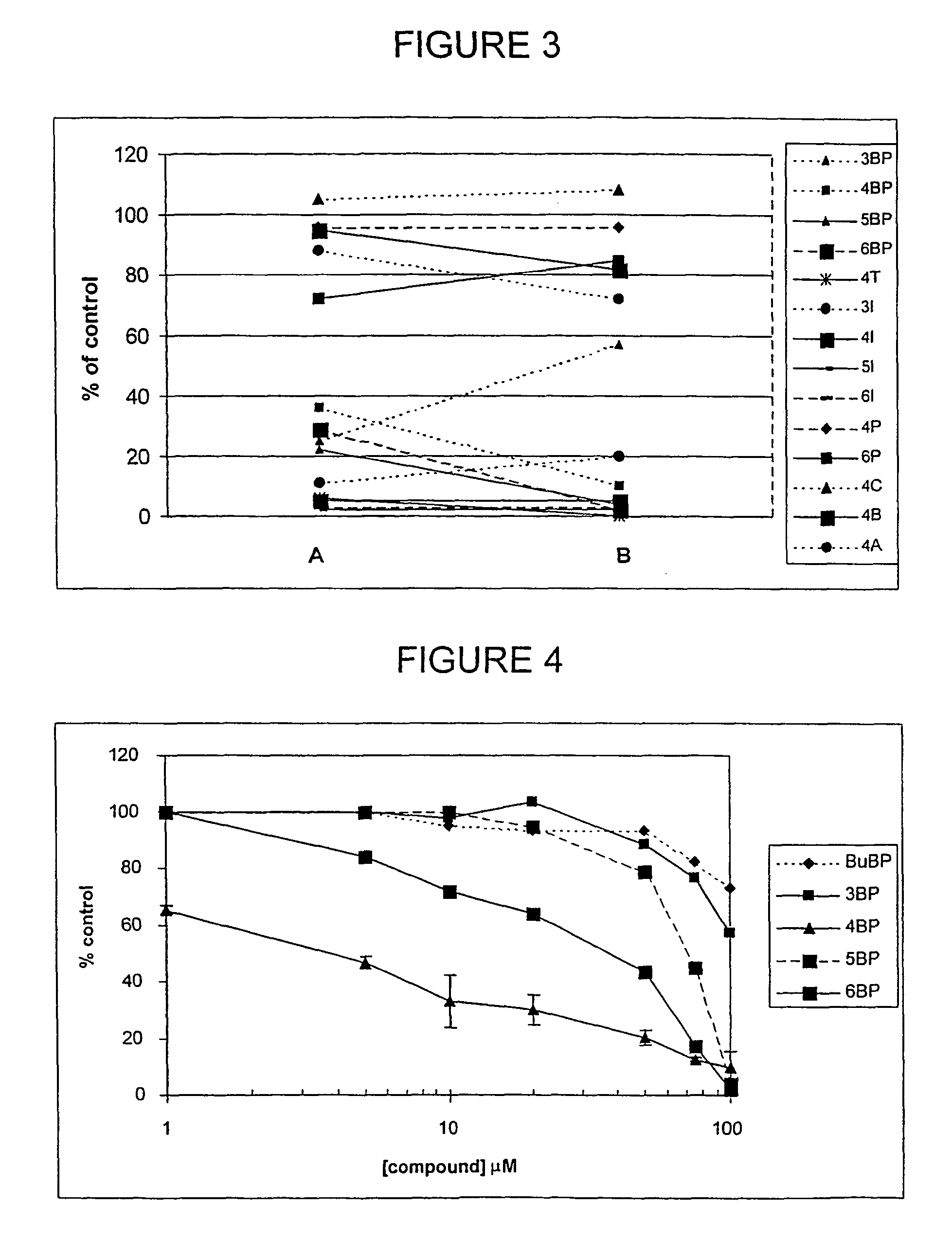
Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds
InactiveUS20090325945A1Promote apoptosisInhibiting cell cycle progressionBiocideOrganic chemistryDiseaseMelanoma
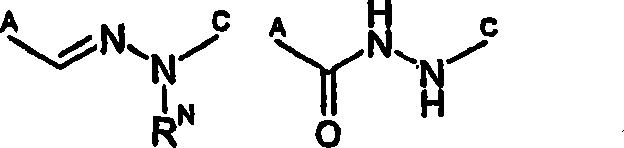
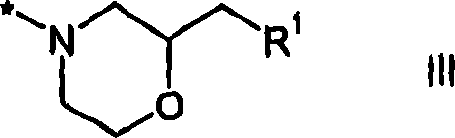
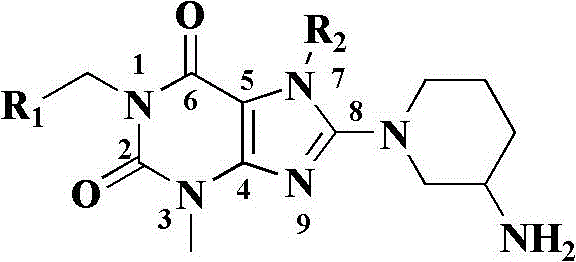
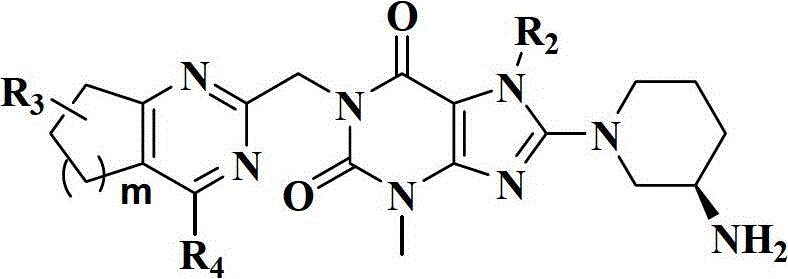
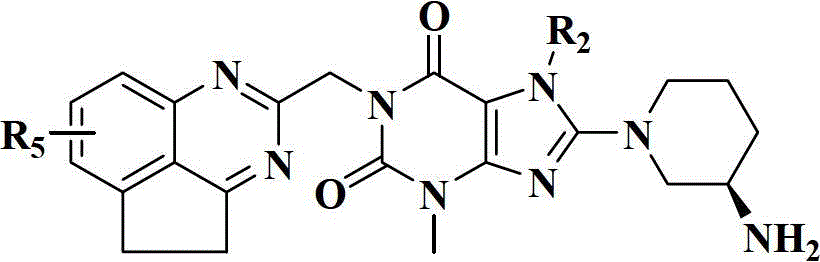
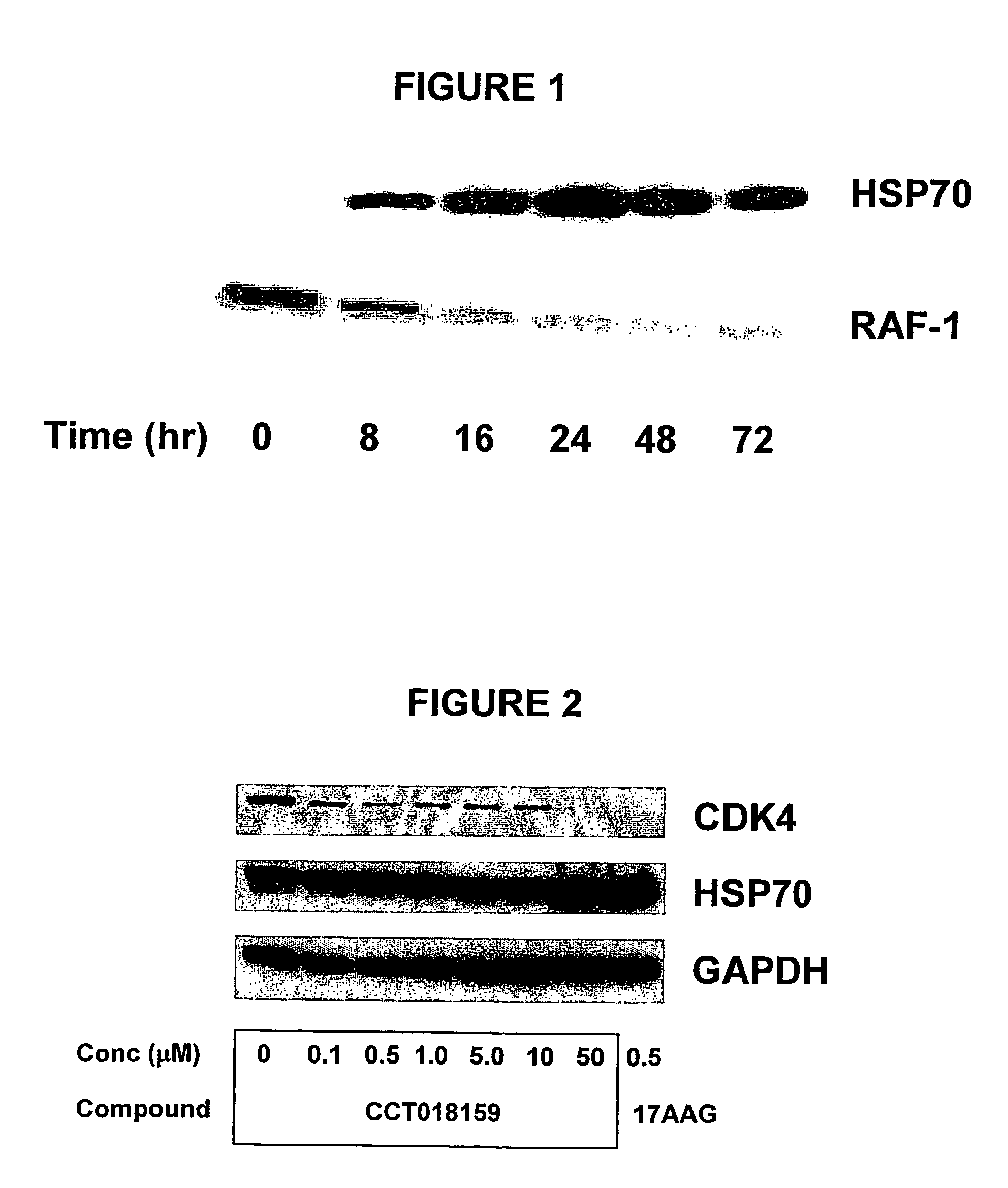
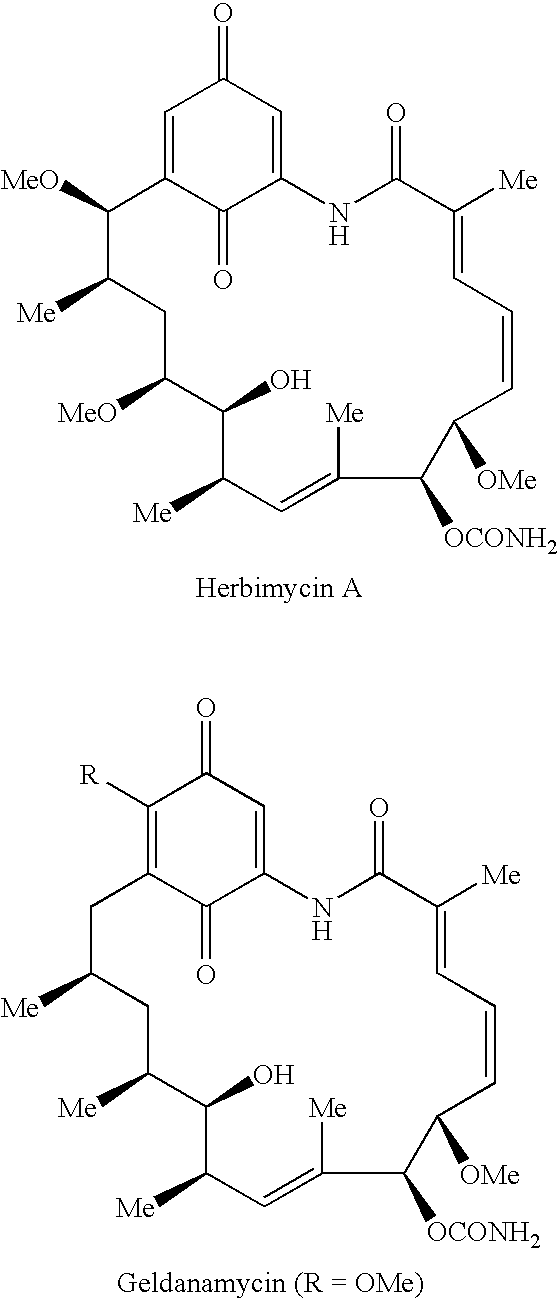
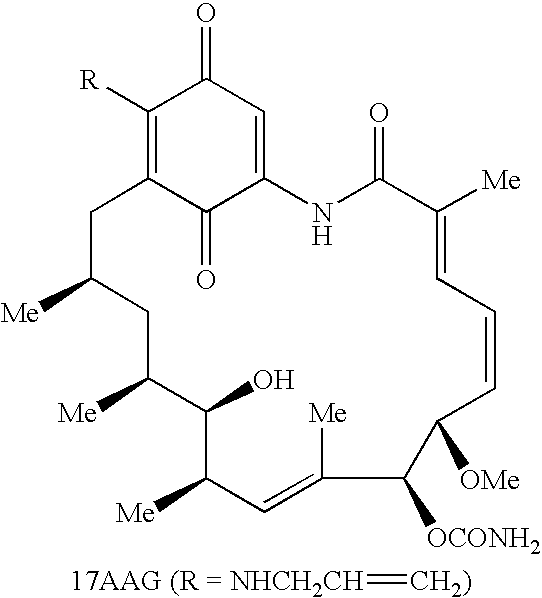
The present invention pertains to certain imidazo[4,5-b]pyridin-2-one and oxazolo[4,5 b]pyridin-2-one compounds and analogs thereof, which, inter alia, inhibit RAF (e.g., B RAF) activity, inhibit cell proliferation, treat cancer, etc., and more particularly to compounds of the formulae: wherein: J is independently —O— or —NRN1−; RN1, if present, is independently —H or a substituent; RN2 is independently —H or a substituent; Y is independently —CH═ or —N═; Q is independently —(CH2)j-M-(CH2)k— wherein: j is independently 0, 1 or 2; k is independently 0, 1, or 2; j+k is 0, 1, or 2; M is independently O—, —S—, —NH—, —NMe-, or —CH2—; each of RP1, RP2, RP5, and RP4 is independently —H or a substituent; and additionally RP1 and RP2 taken together may be CH═CH—CH═CH—; and additionally RP1 and RP5 taken together may be CH═CH—CH═CH—; L is independently: a linker group formed by a chain of 2, 3, or 4 linker moieties; each linker moiety is independently CH2—, —NRN—, —C(═X)—, or —S(═O)2—; either: exactly one linker moiety is —NRN—, or: exactly two linker moieties are —NRN—; either: exactly one linker moiety is —C(═X)—, and no linker moiety is —S(═O)2—, or: exactly one linker moiety is —S(═O)2—, and no linker moiety is —C(═X)—; no two adjacent linker moieties are —NRN—; X is independently ═O or ═S; each RN is independently —H or a substituent; A is independently: C6-14carboaryl, C5-14heteroaryl, C3-12carbocyclic, C3-12heterocyclic; and is independently unsubstituted or substituted; and pharmaceutically acceptable salts, solvates, amides, esters, ethers, N-oxides, chemically protected forms, and prodrugs thereof. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit RAF (e.g., B-RAF) activity, to inhibit receptor tyrosine kinase (RTK) activity, to inhibit cell proliferation, and in the treatment of diseases and conditions that are ameliorated by the inhibition of RAF, RTK, etc., proliferative conditions such as cancer (e.g., colorectal cancer, melanoma), etc.
Owner:CANCER RES TECH LTD +1
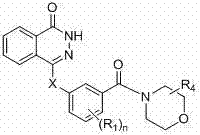
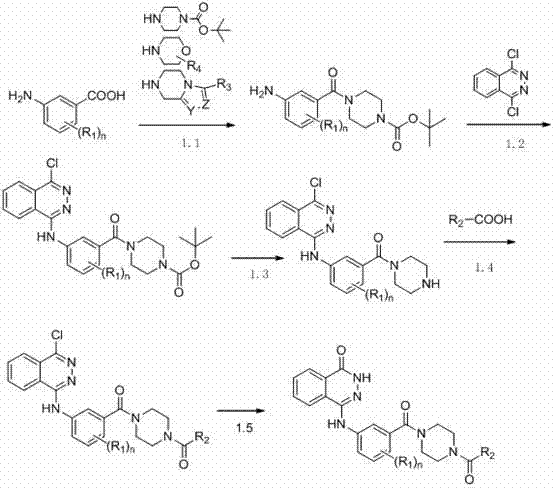
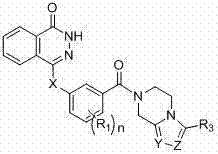
Novel phthalazinone derivatives and uses thereof
The present invention provides novel phthalazinone compounds and isomer thereof, pharmaceutically acceptable salts, solvates, chemically protected forms, and prodrugs; which can be used as PARP inhibitor and pharmaceutical compositions containing the novel phthalazinone compounds; wherein A, R1 and X are defined as shown. The medicine is used for the treatment of: vascular diseases, neurotoxicity, or diseases improved through the inhibition of PARP activity; or used as adjuvants for the treatment of cancers, or used for enhancing the therapeutic effect of radiation or chemotherapeutic agents on tumor cells, wherein the cancers includes breast cancer, ovarian cancer, colon cancer, melanoma, lung cancer, gastrointestinal stromal tumor, brain cancer, cervical cancer, pancreatic cancer, prostate cancer, gastric cancer, chronic myeloid leukocytes hypercytosis, liver canser, lymphoma, peritoneal cancer, soft tissue sarcoma, neuroendocrine tumors, advanced solid tumors, and glioblastoma.
Owner:NANJING SANHOME PHARMACEUTICAL CO LTD
Carbamic acid compounds comprising a piperazine linkage as hdac inhibitors
InactiveUS20050143385A1Promote apoptosisInhibiting cell cycle progressionOrganic active ingredientsNervous disorderAcetylase activityProdrug
This invention pertains to certain carbamic acid compounds which inhibit HDAC (histone deacetylase) activity of the following formula: wherein: Cy is independently a cyclyl group; Q1 is independently a covalent bond or cyclyl leader group; the piperazin-1,4-diyl group is optionally substituted; J1 is independently a covalent bond or —C(═;O)—; J2 is independently —C(═O)— or —S(═O)2—; Q2 is independently an acid leader group; wherein: Cy is independently: C3-20carbocyclyl, C3-20heterocyclyl, or C5-20aryl; and is optionally substituted; Q1 is independently: a covalent bond; C1-7alkylene; or C1-7alkylene-X—C1-7alkylene, —X—C1-7alkylene, or C1-7alkylene-X—, wherein X is —O— or —S—; and is optionally substituted; Q2 is independently: C4-8alkylene; and is optionally substituted; and has a backbone length of at least 4 atoms; or: Q2 is independently: C5-20arylene; C5-20arylene-C1-7alkylene; C1-7alkylene-C5-20arylene; or, C1-7alkylene-C5-20arylene-C1-7alkylene; and is optionally substituted; and has a backbone length of at least 4 atoms; or a pharmaceutically acceptable salt, solvate, amide, ester, ether, chemically protected form, or prodrug thereof. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit HDAC, and in the treatment of conditions mediated by HDAC, cancer, proliferative conditions, psoriasis, etc.
Owner:TOPOTARGET UK LTD
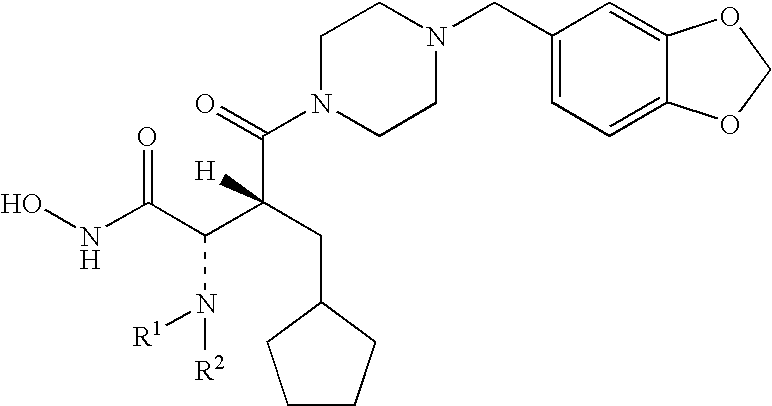
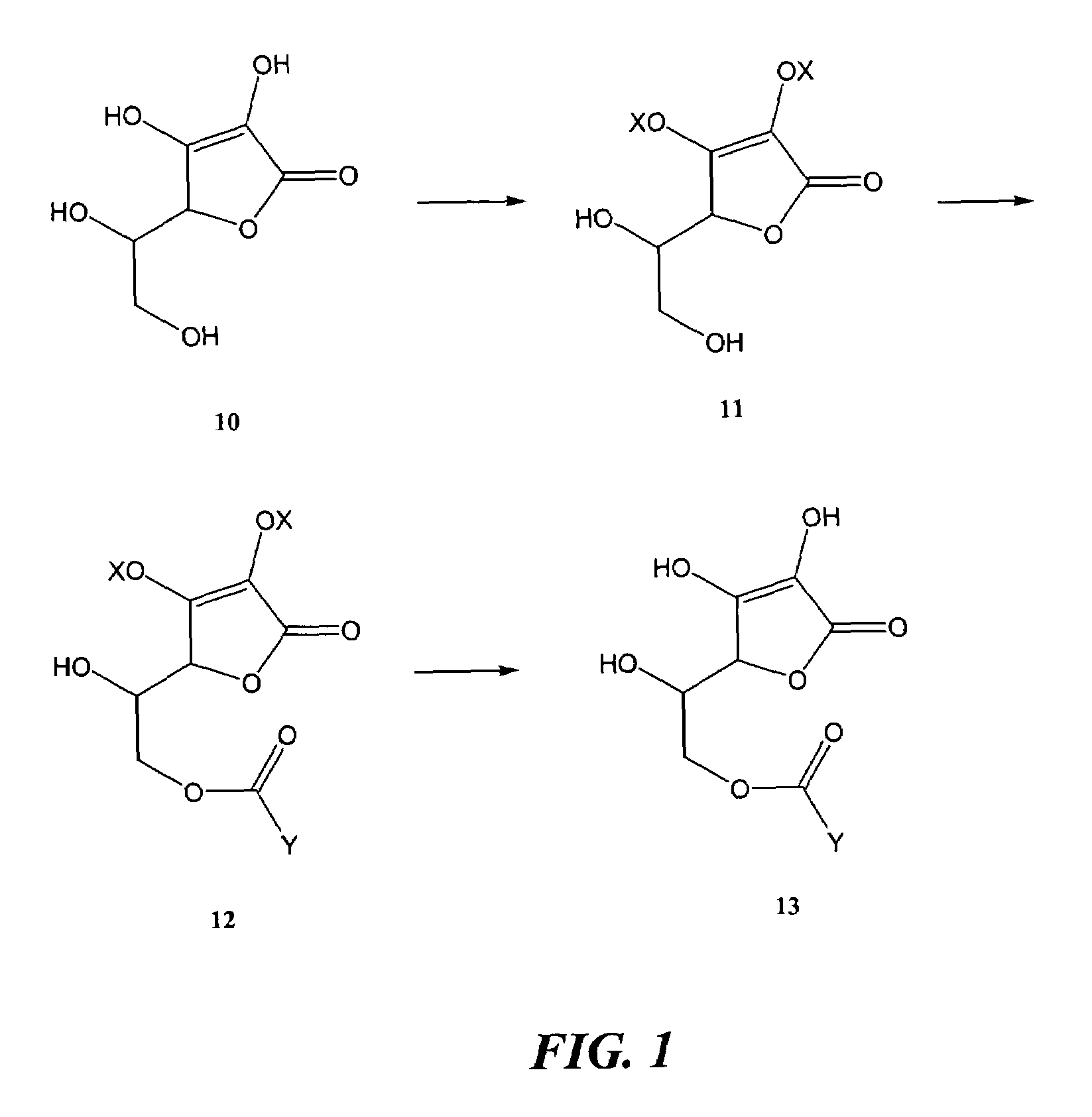
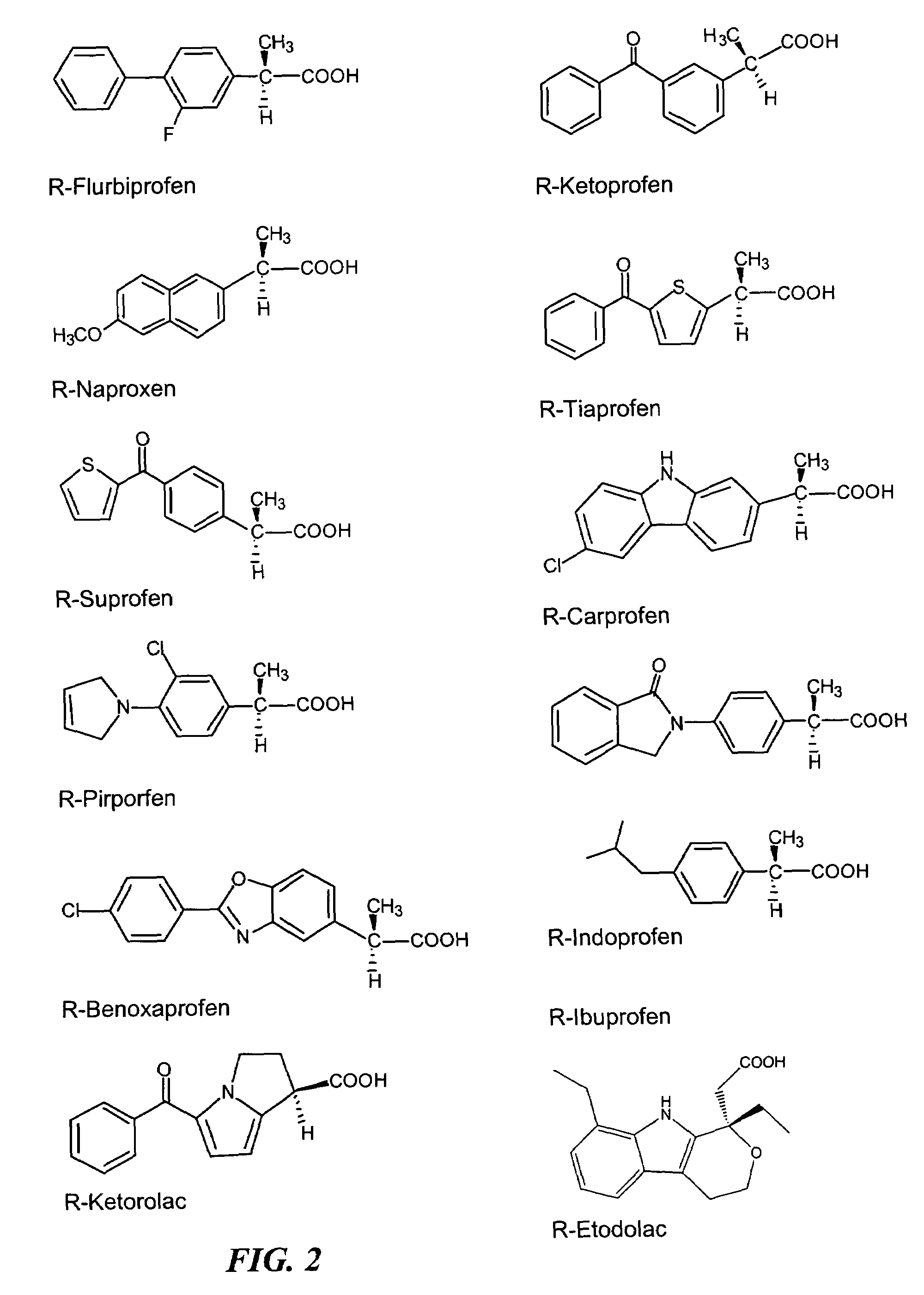
R-NSAID esters and their use
The present invention concerns esters of R-enantiomers of a non-steroidal anti-inflammatory drug, which is substantially free from the S-enantiomer. The compounds of the invention may be used in treating a disease or illness in a mammal. To this end, a composition comprising a compound mentioned above, or a pharmaceutically acceptable salt thereof where salt formation occurs, is administered to the mammal in an amount sufficient to elicit a chemopreventative effect or a chemoprotective effect or a therapeutic effect or a prophylactic effect.
Owner:ENCORE PHARMA
3,4-diarylpyrazoles and their use in the therapy of cancer
The present invention pertains to the use of certain 3,4-diarylpyazoles of formula (I), both in vitro and in vivo, to inhibit heat shock protein 90 (HSP90), and in the treatment of conditions mediated by HSP90, including, for example, cancer; wherein: Ar3 is independently: a C5-20aryl group, and is optionally substituted; Ar4 is independently: a C5-20aryl group, and is optionally substituted; R5 is independently: hydrogen; halo; hydroxyl; ether; formyl; acyl; carboxy; ester; acyloxy; oxycarbonyloxy; amido; acylamido; aminocarbonyloxy; tetrazolyl; amino; nitro; cyano; azido; sulfhydryl; thioether; sulfonamide; C1-7alkyl; C3-20heterocycyl; or C5-20aryl; R<SP>N< / SP> is independently: —H; C1-7alkyl; C3-20heterocycyl; or, C5-20aryl; and pharmaceutically acceptable salts, solvates, amides, esters, ethers, chemically protected forms, and prodrugs thereof. The present invention also pertains to such compounds, pharmaceutical compositions comprising such compounds, such compounds for medical use, such compounds use in the treatment of conditions mediated by HSP90, including, for example, cancer, and use of such compounds in the preparation of medicaments for such treatments.
Owner:VERNALIS (R&D) LTD +2
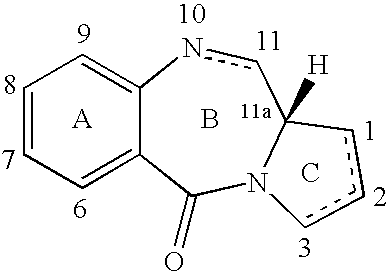
Pyrrolobenzodiazepines as key intermediates in the synthesis of dimeric cytotoxic pyrrolobenzodiazepines
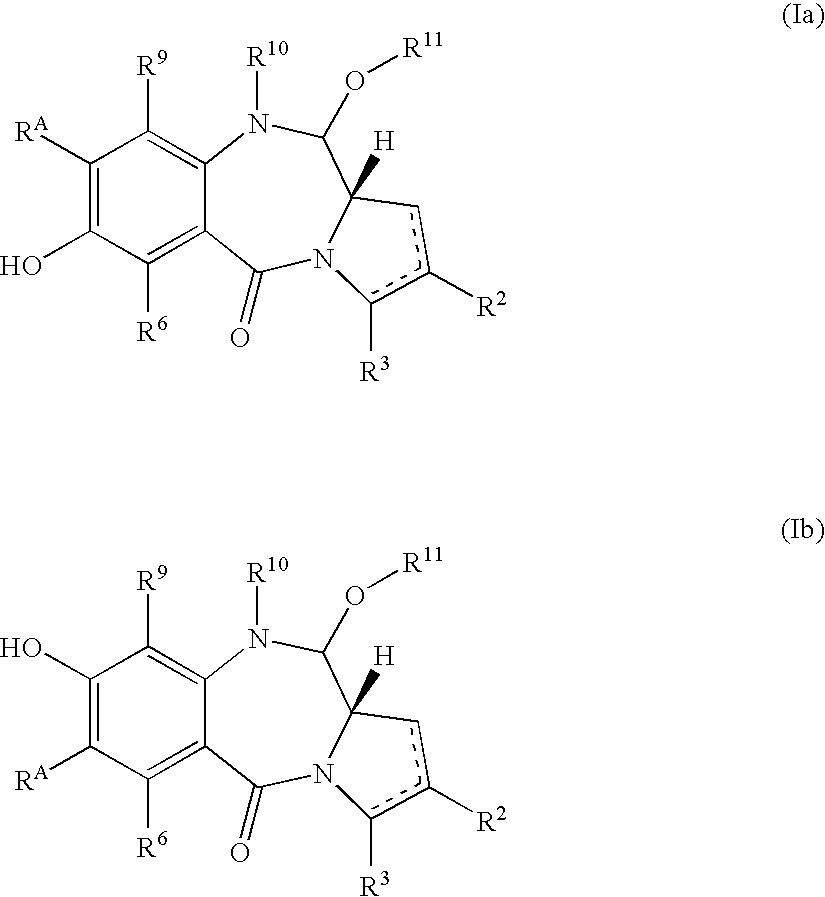
Compounds and a method of synthesis of compounds of formula (Ia) or (Ib): and salts, solvates, and chemically protected forms thereof, wherein the dotted lines indicate the optional presence of a double bond between C1 and C2 or C2 and C3; R2 and R3 are independently selected from —H, ═O, ═CH2, —CN, —R, OR, halo, ═CH—R, O—SO2—R, CO2R and COR; R10 is a carbamate-based nitrogen protecting group; and R11 is an oxygen protecting group.
Owner:MEDIMMUNE LTD
Alkane diol derivatives as therapeutic agents for the treatment of bone conditions
The present invention pertains to certain alkane diol derivatives (including, e.g., mono- and di-esters) of the formula R1—O-A-O—R2, wherein: A is a C2-10 alkylene group; R1 is independently a first hydroxy protecting group (e.g., an ester group); and, R2 is independently —H or a second hydroxy protecting group (e.g., an ester group); and pharmaceutically acceptable salts, solvates, amides, esters, ethers, chemically protected forms, and prodrug thereof, which, inter alia, inhibit osteoclast survival, formation, and / or activity; and / or inhibit bone resorption. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit osteoclast survival, formation, and / or activity, and to inhibit conditions mediated by osteoclasts and / or characterised by bone resorption, such as osteoporosis, rheumatoid arthritis, cancer associated bone disease, Paget's disease, and the like; and / or conditions associated with inflammation or activation of the immune system.
Owner:THE UNIV COURT OF THE UNIV OF ABERDEEN REGENT WALK
Imidazo[4,5-b]pyridin-2-one and oxazolo[4,5-b]pyridin-2-one compounds and analogs thereof as therapeutic compounds
The present invention pertains to certain imidazo[4,5-b]pyridin-2-one and oxazolo[4,5-b]pyridin-2-one compounds and analogs thereof, which, inter alia, inhibit RAF (e.g., B-RAF) activity, inhibit cell proliferation, treat cancer, etc., and more particularly to compounds of the formula (I): wherein: J is independently —O— or —NRN1—; RN1, if present, is independently —H or a substituent; RN2 is independently —H or a substituent; Y is independently —CH═ or —N═; Q is independently —(CH2)j—M—(CH2)k— wherein: j is independently 0, 1 or 2; k is independently 0, 1, or 2; j+k is 0, 1, or 2; M is independently —O—, —S—, —NH—, —NMe—, or —CH2—; each of RP1, RP2, RP3, and RP4 is independently —H or a substituent; and additionally RP1 and RP2 taken together may be —CH═CH—CH═CH—; L is independently: a linker group formed by a chain of 2, 3, or 4 linker moieties; each linker moiety is independently —CH2—, —NRN—, —C(═X)—, or —S(═O)2—; exactly one linker moiety is —NRN—, or: exactly two linker moieties are —NRN—; exactly one linker moiety is —C(═X)—, and no linker moiety is —S(═O)2—; or: exactly one linker moiety is —S(═O)2—, and no linker moiety is —C(═X)—; no two adjacent linker moieties are —NRN—; X is independently ═O or ═S; each RN is independently —H or a substituent; A is independently: C6-14carboaryl, C5-14heteroaryl, C3-12carbocyclic, C3-12heterocyclic; and is independently unsubstituted or substituted; and pharmaceutically acceptable salts, solvates, amides, esters, ethers, N-oxides, chemically protected forms, and prodrugs thereof. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit RAF (e.g., B-RAF) activity, to inhibit receptor tyrosine kinase (RTK) activity, to inhibit cell proliferation, and in the treatment of diseases and conditions that are ameliorated by the inhibition of RAF, RTK, etc., proliferative conditions such as cancer (e.g., colorectal cancer, melanoma), etc.
Owner:THE INST OF CANCER RES ROYAL CANCER HOSPITAL +2
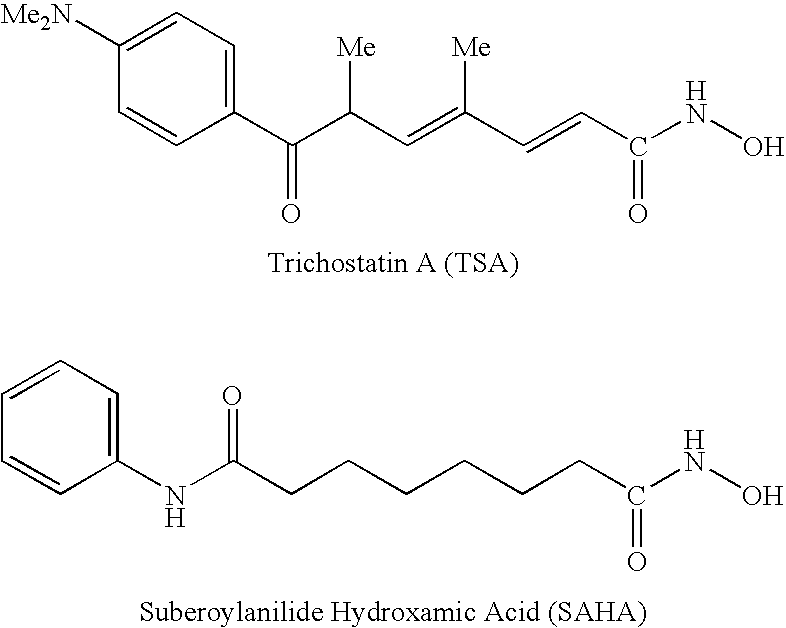
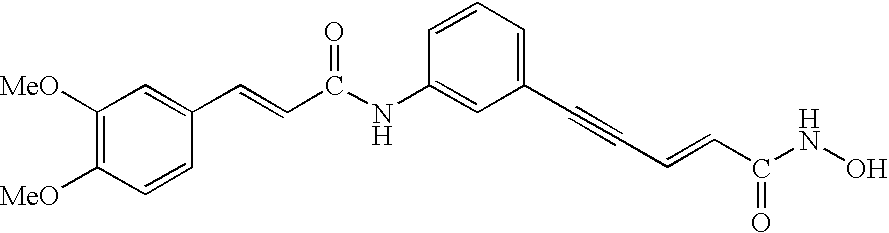
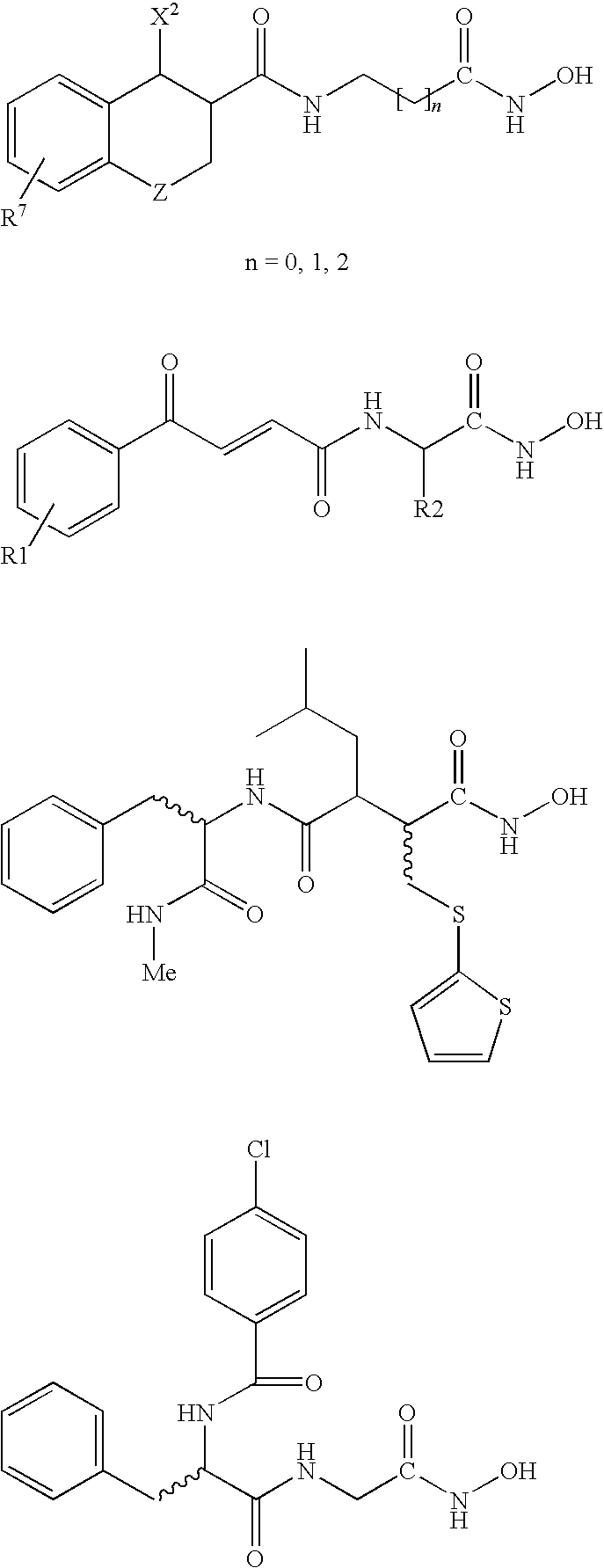
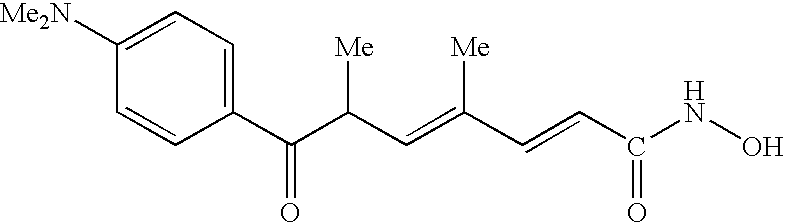
Carbamic acid compounds comprising an amide linkage as HDAC inhibitors
This invention pertains to certain active carbamic acid compounds which inhibit HDAC activity and which have the formula (1) wherein: A is an aryl group; Q1 is an aryl leader group having a backbone of at least 2 carbon atoms; J is an amide linkage selected from: —NR1C(═O)— and —C(═O)NR1—; R1 is an amido substituent; and, Q2 is an acid leader group; and pharmaceutically acceptable salts, solvates, amides, esters, ethers, chemically protected forms, and prodrugs thereof. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit HDAC, and, e.g., to inhibit proliferative conditions, such as cancer and psorias
Owner:TOPOTARGET UK LTD
Carbamic acid compounds comprising an amide linkage as HDAC inhibitors
This invention pertains to certain active carbamic acid compounds which inhibit HDAC activity and which have the formula (1) wherein: A is an aryl group; Q1 is an aryl leader group having a backbone of at least 2 carbon atoms; J is an amide linkage selected from: —NR1C(═O)— and —C(═O)NR1-; R1 is an amido substituent; and, Q2 is an acid leader group; and pharmaceutically acceptable salts, solvates, amides, esters, ethers, chemically protected forms, and prodrugs thereof. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit HDAC, and, e.g., to inhibit proliferative conditions, such as cancer and psoriasis.
Owner:TOPOTARGET UK LTD
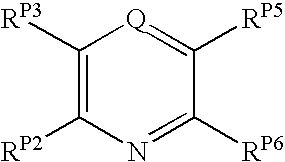
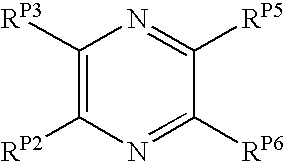
Pyrazines and Pyridines and Derivatives Thereof as Therapeutic Compounds
InactiveUS20080015191A1Promote apoptosisInhibiting cell cycle progressionAntibacterial agentsOrganic active ingredientsDiseaseMelanoma
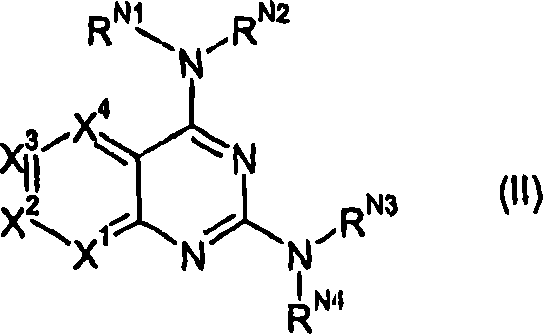
The present invention pertains to certain pyrazines and pyridines, and derivatives thereof, which, inter alia, inhibit RAF (e.g., B RAF) activity, inhibit cell proliferation, treat cancer, etc., and more particularly to compounds of the formulae: (I) wherein: Q is independently —N═ or —CH═; one of RP2 and RP3 is independently a group of the formula -J1-L1-Z; wherein: if Q is —N═, then -J1-L1-Z is independently: —NH-Z; —O-Z; or S-Z; if Q is —CH═, then -J1-L1-Z is independently: —NH—(CH2)n-Z, wherein n is independently 0 or 1; —O-Z; or —S-Z; Z is independently: C6-14 carboaryl, C5-14 heteroaryl, C3-12carbocyclic, C3-12 heterocyclic; and is independently unsubstituted or substituted; the other of RP2 and RP3 is independently —H, —NHRN1, or —NHC(═O)RN2; wherein: RN1, if present, is independently —H or aliphatic saturated C1-4alkyl; RN2, if present, is independently —H or aliphatic saturated C1-4alkyl; one of RP5 and RP6 is independently a group of the formula —W—Y; wherein: W is independently: a covalent bond; —NRN4—, —O—, —S—, —C(═O)—, —CH2—; —NRN4—CH2—, —O—CH2—, —S—CH2—, —C(═O)—CH2—, —(CH2)2—; —CH2—NRN4—, —CH2—O—, —CH2—S—, or —CH2—C(═O)—; wherein RN4, if present, is independently —H or aliphatic saturated C1-4alkyl; Y is independently: C6-14carboaryl, C5-14heteroaryl, C3-12carbocyclic, C3-12heterocyclic; and is independently unsubstituted or substituted; the other of RP5 and RP6 is independently —H; and pharmaceutically acceptable salts, solvates, amides, esters, ethers, N-oxides, chemically protected forms, and prodrugs thereof. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, e.g., both in vitro and in vivo, to inhibit RAF (e.g., B-RAF) activity, to inhibit receptor tyrosine kinase (RTK) activity, to inhibit cell proliferation, and in the treatment of diseases and conditions that are ameliorated by the inhibition of RAF, RTK, etc., proliferative conditions such as cancer (e.g., colorectal cancer, melanoma), etc.
Owner:THE WELLCOME TRUST TRUSTEE TO THE WELLCOME TRUST
Hydrazinomethyl, hydr zonomethyl and 5-membered heterocyclic compounds which act as mTOR inhibitors and their use as anti cancer agents
InactiveCN101128440AOrganic chemistryHeterocyclic compound active ingredientsCarboxyl radicalAnticarcinogen
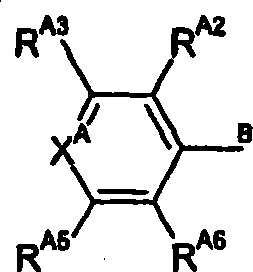
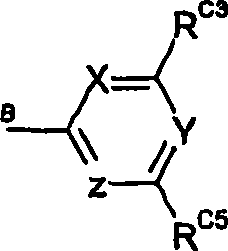
Compounds of formula (I): A-B-C and isomers, salts, solvates, chemically protected forms, and prodrugs thereof wherein: B is selected from the group consisting of formula (i) where RN is H or Me; or B is a divalent C5 heterocyclic residue containing one or two ring heteroatoms; A is formula (ii) RA3 and RA5 are independently selected from halo, ORO and RAC, where RO is H or Me, and RAC is H or C1-4 alkyl; XA is selected from N and CRA4, where RA4 is selected from H, ORO, CH2OH, CO2H, NHSO2Me and NHCOMe; RA2 and RA6 are independently selected from H, halo and ORO; or RA3 and RA4 together with the carbon atoms to which they are attached, or RA2 and RA3 together with the carbon atoms to which they are attached, may form a C5-6 heterocylic or heteroaromatic ring, containing at least one nitrogen ring atom; where if X is not N, 1 , 2, or 3 of RA2 to RA6 are not H; C is formula (iii) where X is selected from N and CH, Y is selected from N and CH, and Z is selected from N and CRC6; RC3 is selected from H, halo and an optionally substituted N-containing C5-7 heterocyclic group; RC5 is a group selected from formula (iv) which group may be selected by one or two C1-4 alkyl groups or a carboxy group; RC6 is H; or, when X and Y are N, RC5 and RC6 (when Z is CRC6) together with the carbon atoms to which they are attached may form a fused C6 aromatic ring selected from the group consisting of formula (v).
Owner:MAYBRIDGE LTD GB
Oxidation-inhibited biological nutritive healthy liquid and preparing method thereof
InactiveCN1472301AStrong bloodthirsty vitalityPurify fermentation systemAlcoholic beverage preparationFood scienceDiseaseOrganism
An antioxidizing health-care nutritive liquid is prepared from haw and red sage root and features use of saccharomyces cerevisiae (CGMCC No.0775) for high-purity fermentation. Its advantage is its high health-care actions of removing free radicals of oxygen, regulating internal secretion, lowering blood sugar, blood fat and blood pressure, preventing and treating cardiovascular and cerebrovascular diseases and cancers, delaying senility and beautifying skin.
Owner:李公甫
2,4-diamino-pyridopyrimidine derivatives and their use as mTOR inhibitors
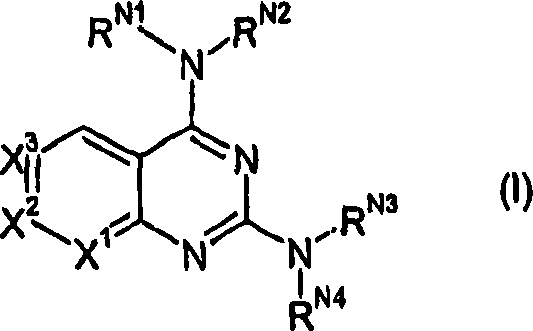
Compounds of formula (I) and isomers, salts, solvates, chemically protected forms, and prodrugs thereof one of X1, X2 and X3 is N, and the others are CH; RN1 and RN2 together with the nitrogen atom to which they are attached form a nitrogen-containing heterocyclic ring having from 4 to 8 ring atoms; RN3 and RN4 together with the nitrogen atom to which they are attached form a nitrogen-containing heterocyclic ring having from 4 to 8 ring atoms and their use in treating diseases ameliorated by the inhibition of mTOR.
Owner:MAYBRIDGE LTD GB
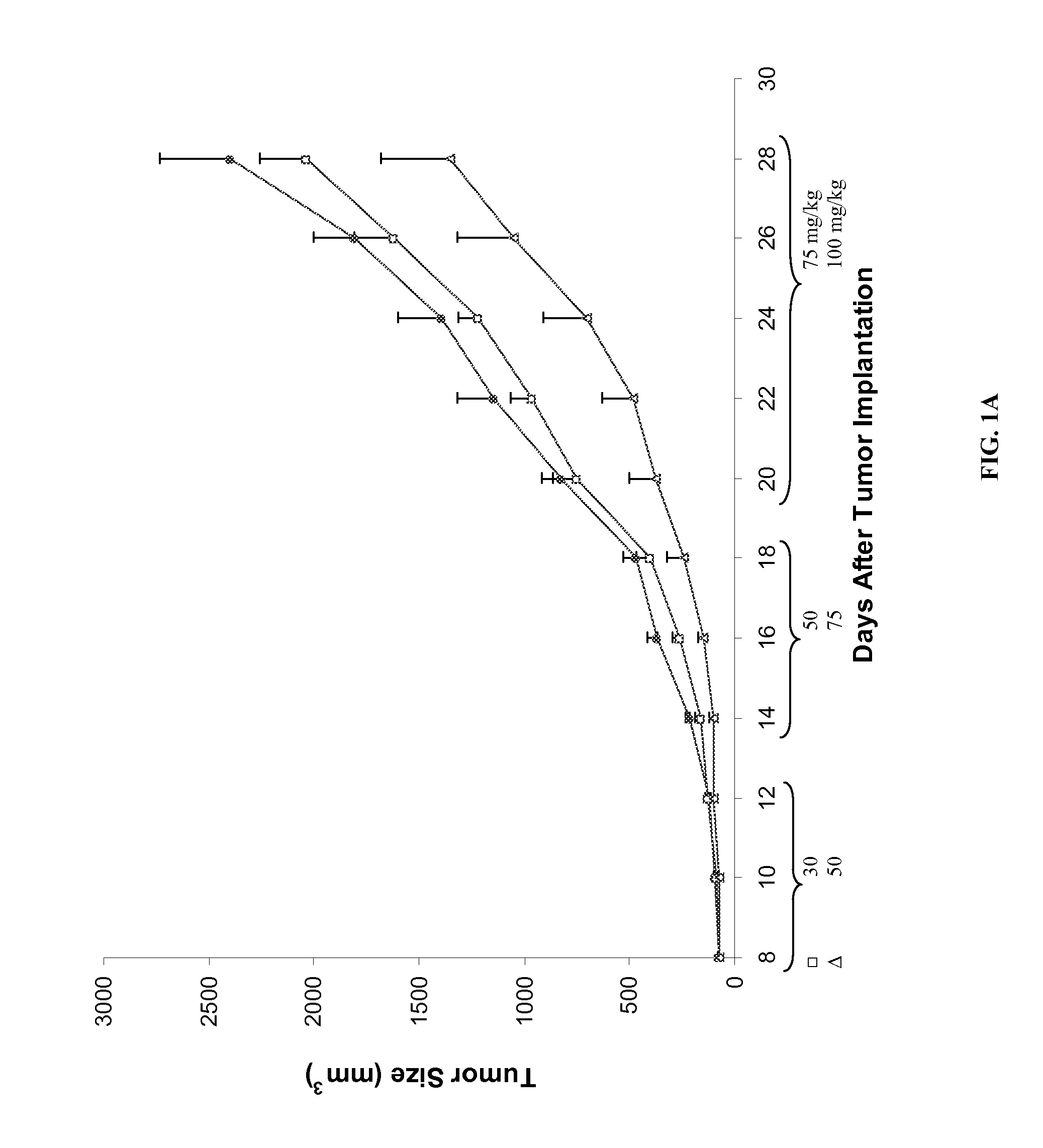
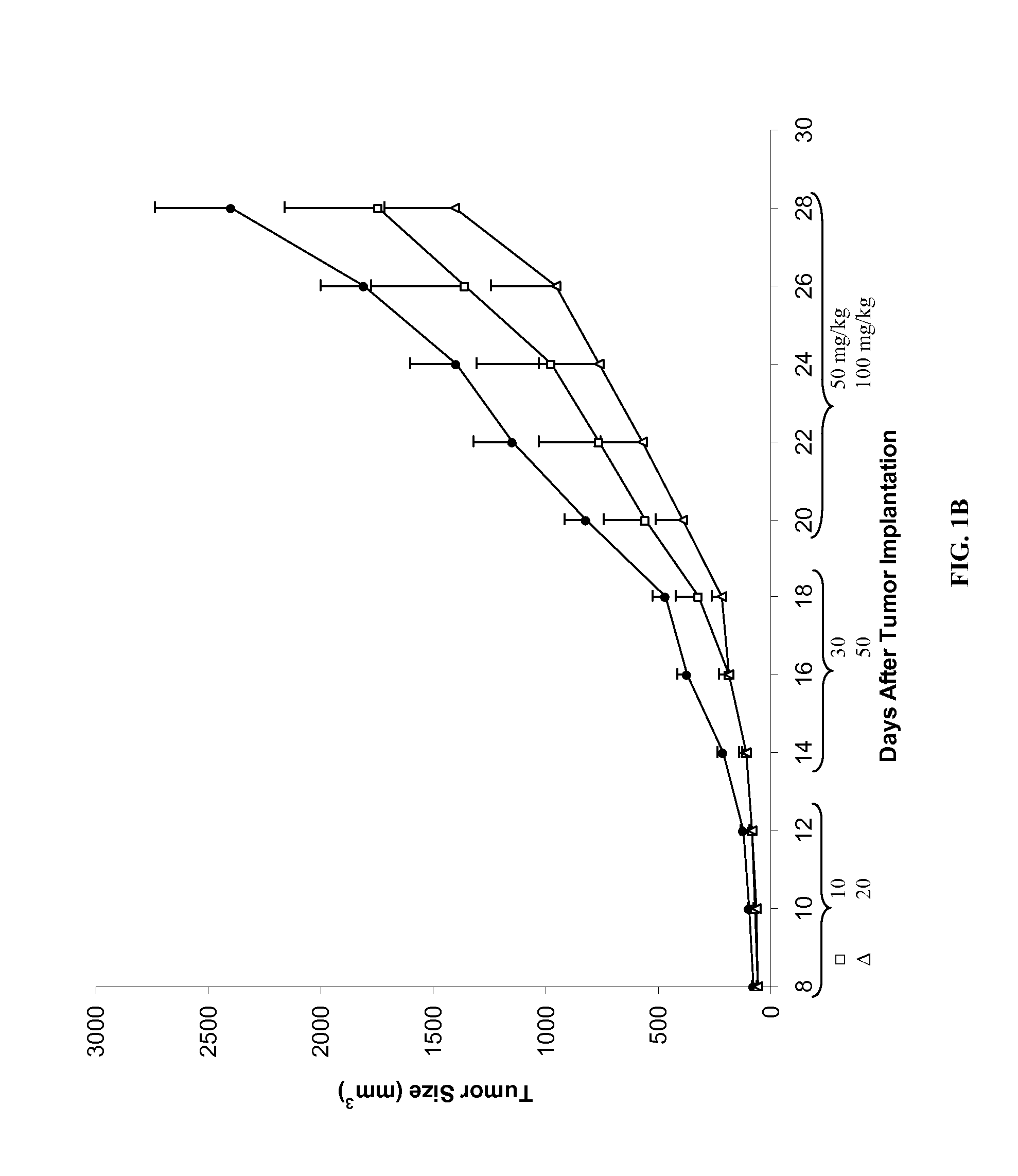
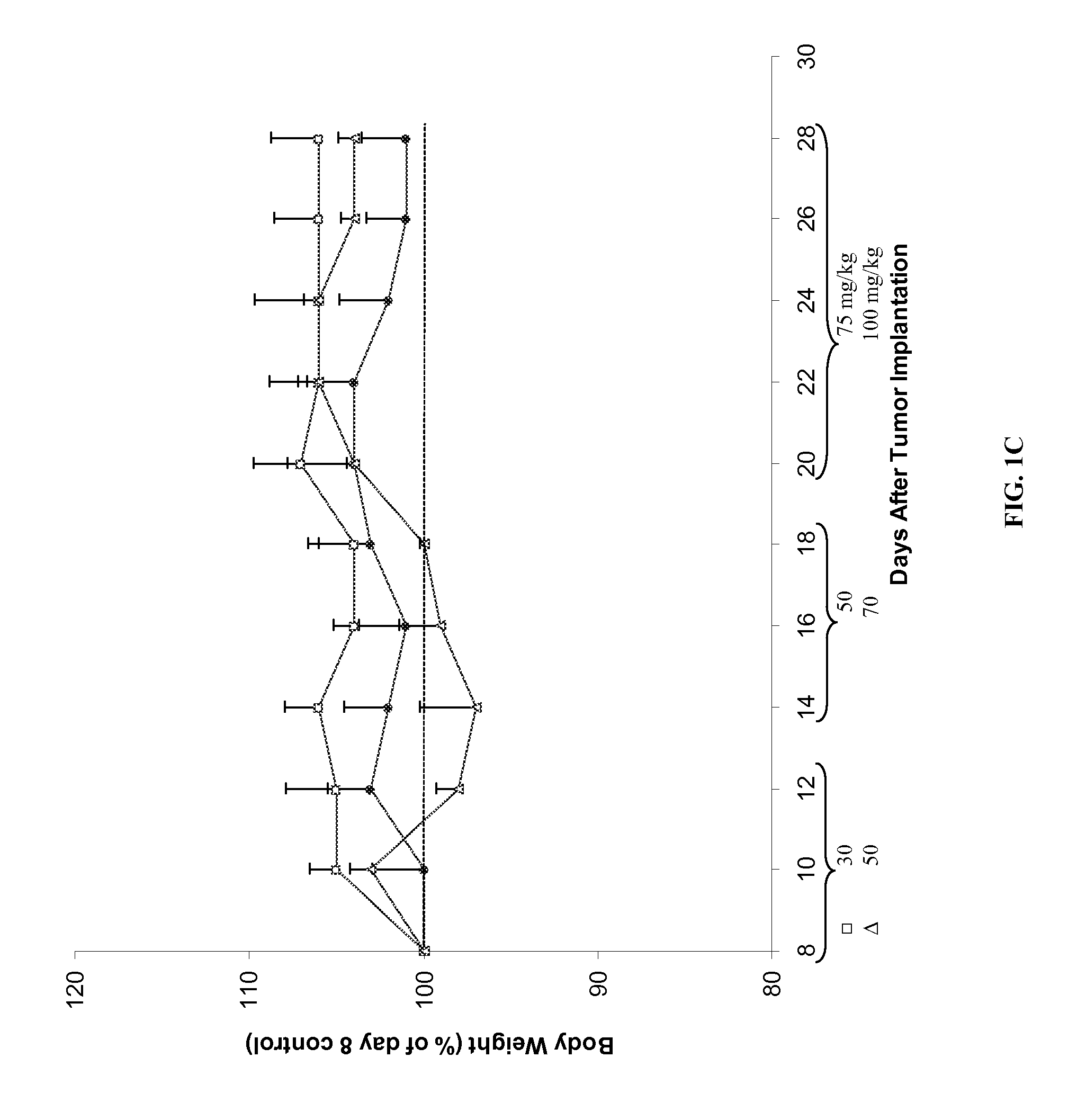
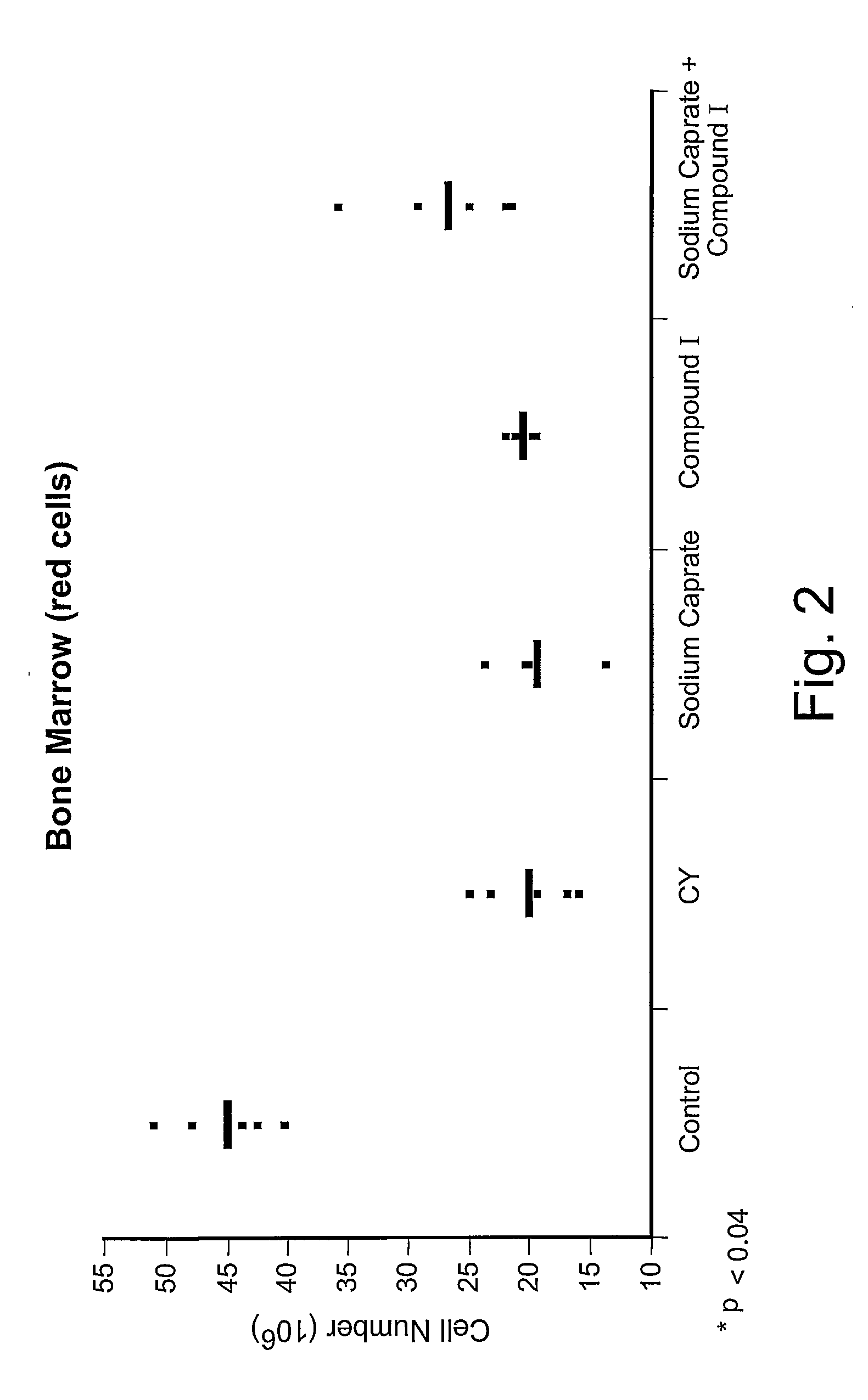
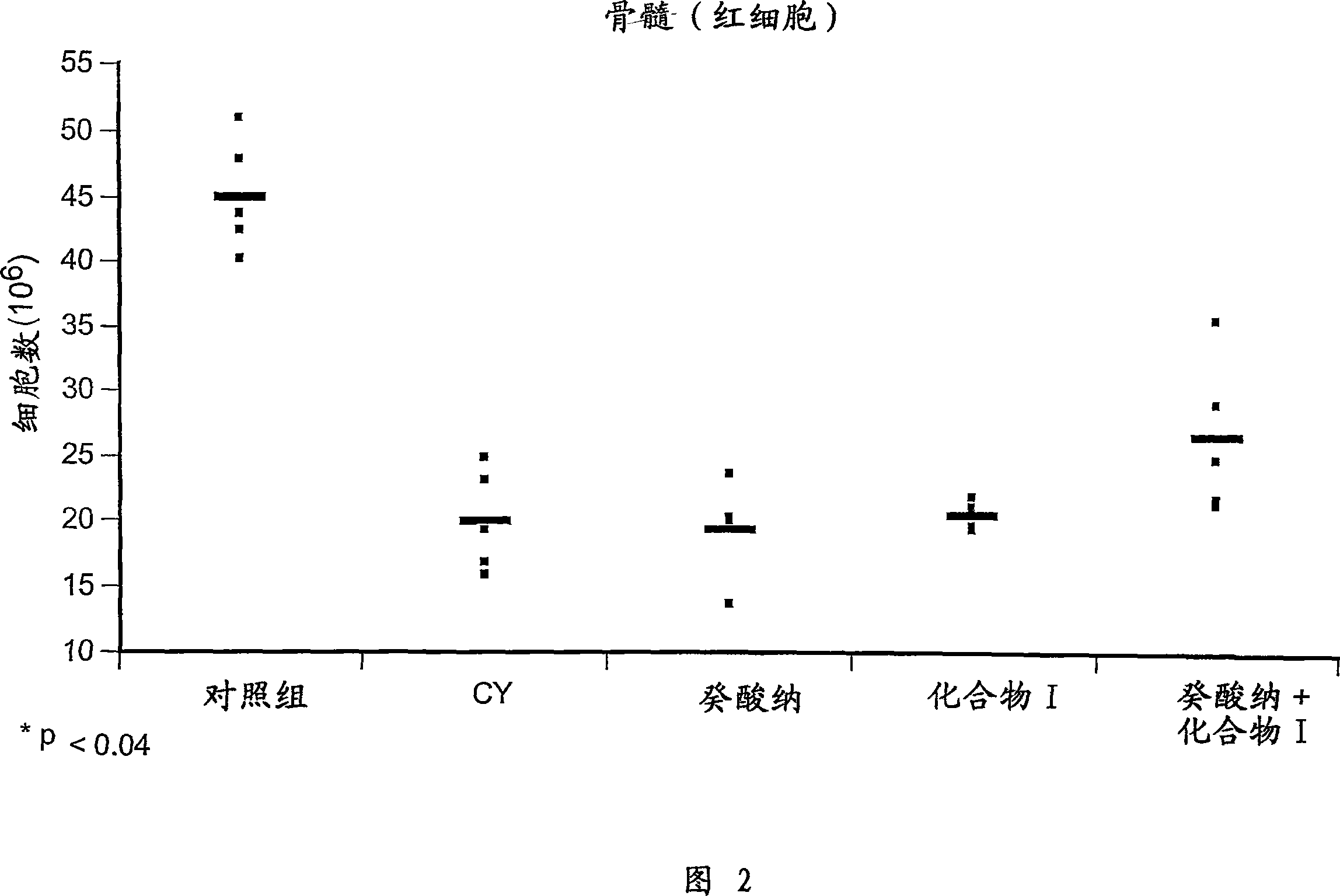
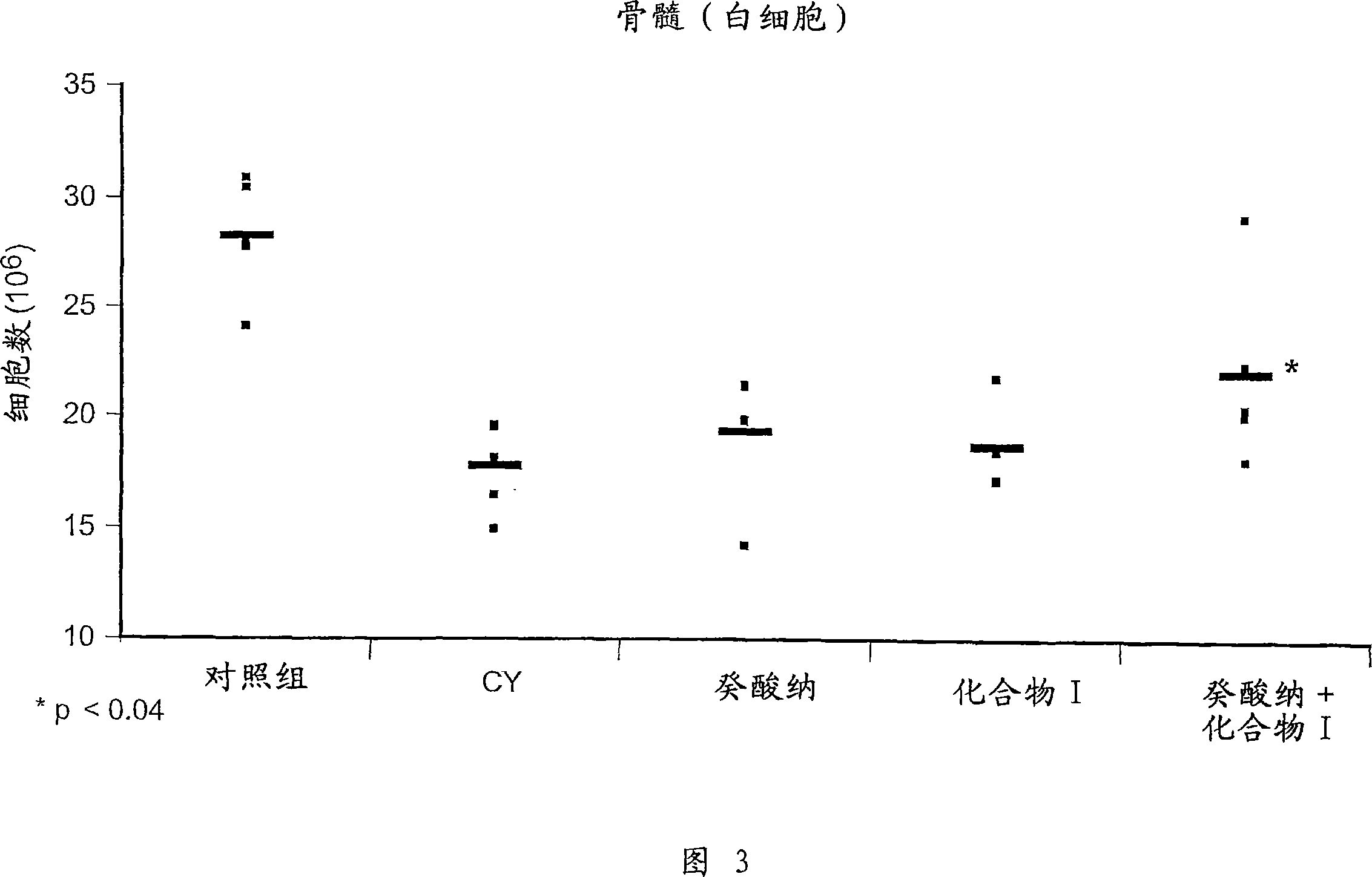
Chemoprotective methods
InactiveUS8143236B2Reduce and prevent and mitigate and delayReducing and preventing and mitigating and delayingBiocidePhosphorous compound active ingredientsChemoprotectivePharmacology
Compositions and methods for reducing, preventing, mitigating, and / or delaying the onset of, attenuating the severity of, and / or hastening the resolution of, for example, one or more chemotherapy-associated toxicities in a subject receiving one or more chemotherapeutic agents. In addition compositions and methods for mitigating or preventing a novel form of chemotherapy-induced peripheral neuropathy are disclosed and claimed.
Owner:BIONUMERIK PHARMA INC
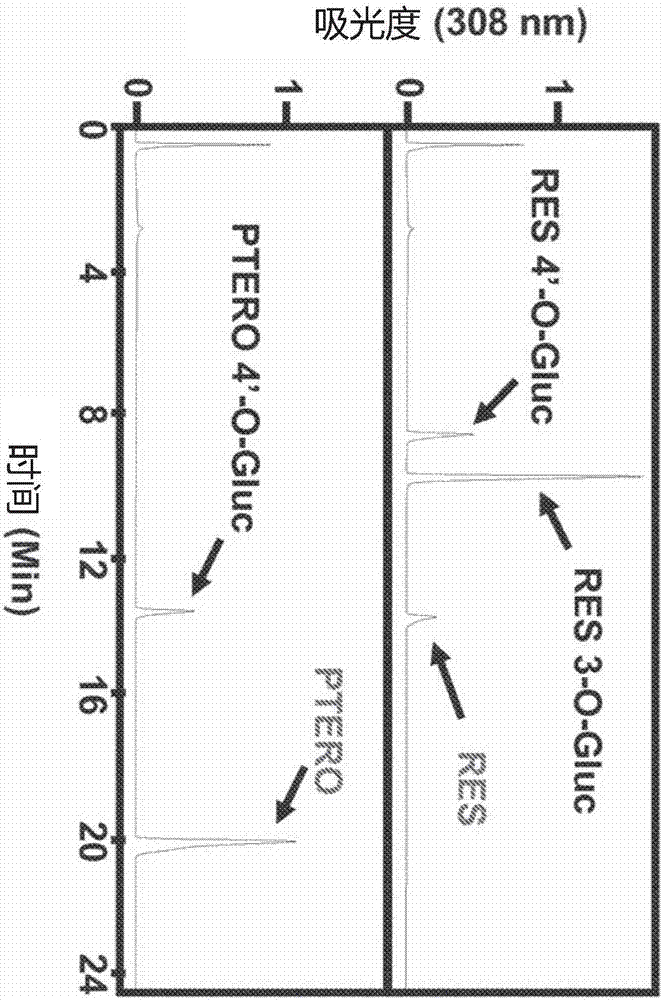
Composition containing resveratrol and/or derivatives thereof and plant oil, process for producing said composition, nutraceutical and/or pharmaceutical product, and method for enhancing the potential of resveratrol
ActiveUS20130040920A1Promote absorptionQuick eliminationBiocideCosmetic preparationsDiseaseChemoprotective
The present invention is a method for obtaining a formulation of resveratrol and rice bran oil. The resulting product in the form of an oil or solid proves to increase the therapeutic potential of resveratrol by the synergistic action of the components of the rice oil.The product obtained through the method of the invention is an active principle which, when incorporated in nutraceutical and / or pharmaceutical compositions, provides antioxidant, anti-inflammatory, antiviral, cardioprotective, neuroprotective and / or cancer chemoprotective action, besides protecting against infections and ischemia, reducing obesity, and preventing illnesses of old age.
Owner:UNIAO BRASILEIRA DE EDUCACAO E ASSISTENCIA MANTENEDORA DA PUC RS
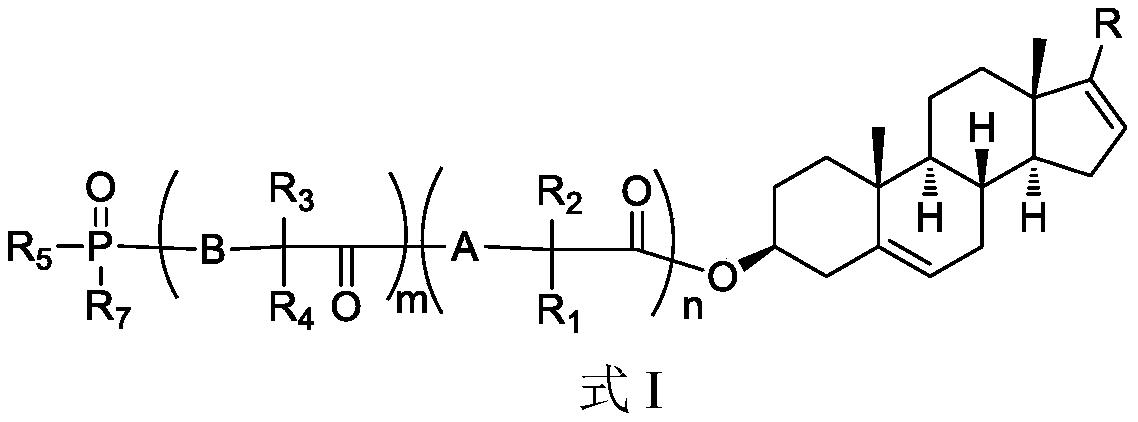
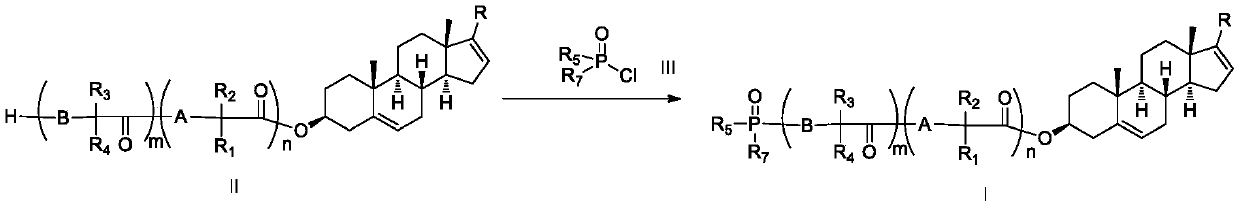
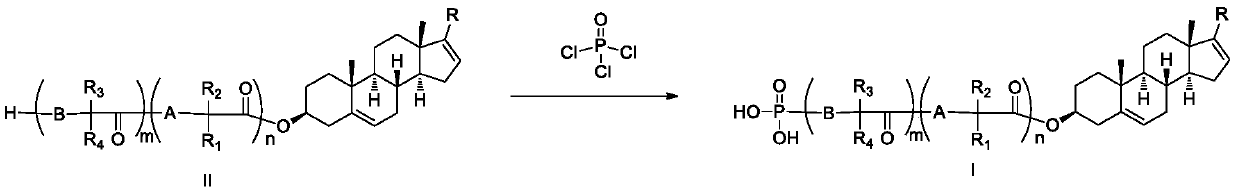
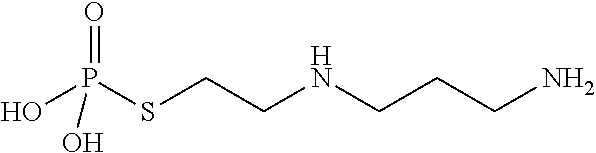
Phosphoryl-containing compounds and application thereof
ActiveCN109879933AEnhanced inhibitory effectHigh anticancer activityOrganic active ingredientsDipeptide ingredientsDiseaseMelanoma
The present invention relates to a series of phosphoryl-containing steroidal compounds, a preparation method and an application thereof, in particular to the compounds as a formula I, an isomer, pharmaceutically acceptable salts or chemically protected forms thereof, the preparation method thereof, and the application of the compounds in preparation of a medicine for treating cancer-related diseases. The compounds of the present invention have the good activity for inhibiting malignant tumors such as melanoma and prostate cancer.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Compounds, compositions and methods for reducing toxicity and treating or preventing diseases
The present invention provides compounds of Formula (I), compositions comprising an effective amount of a compound of Formula (I), optionally with chemotherapeutic drugs such as a tubulin-binding drug, and methods of their use for reducing the toxicity of cytotoxic agents, treating or preventing cancer or a neuropathic disorder, inducing a chemoprotective phase II enzyme, DNA, or protein synthesis, enhancing the immune system, treating inflammation, improving and enhancing general health or well-being, and methods for making compounds of Formula (I).
Owner:DANISHEFSKY SAMUEL J +6
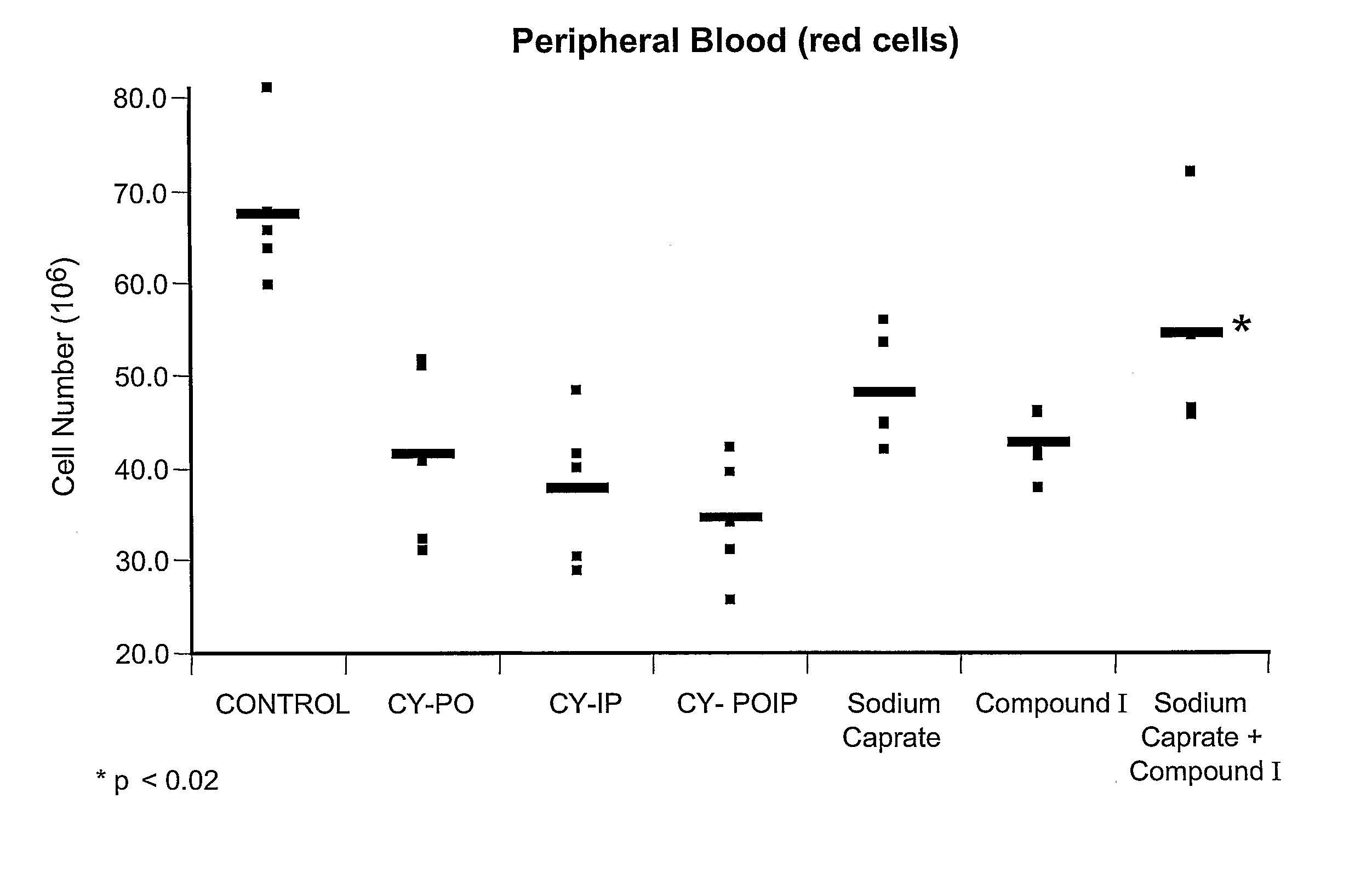
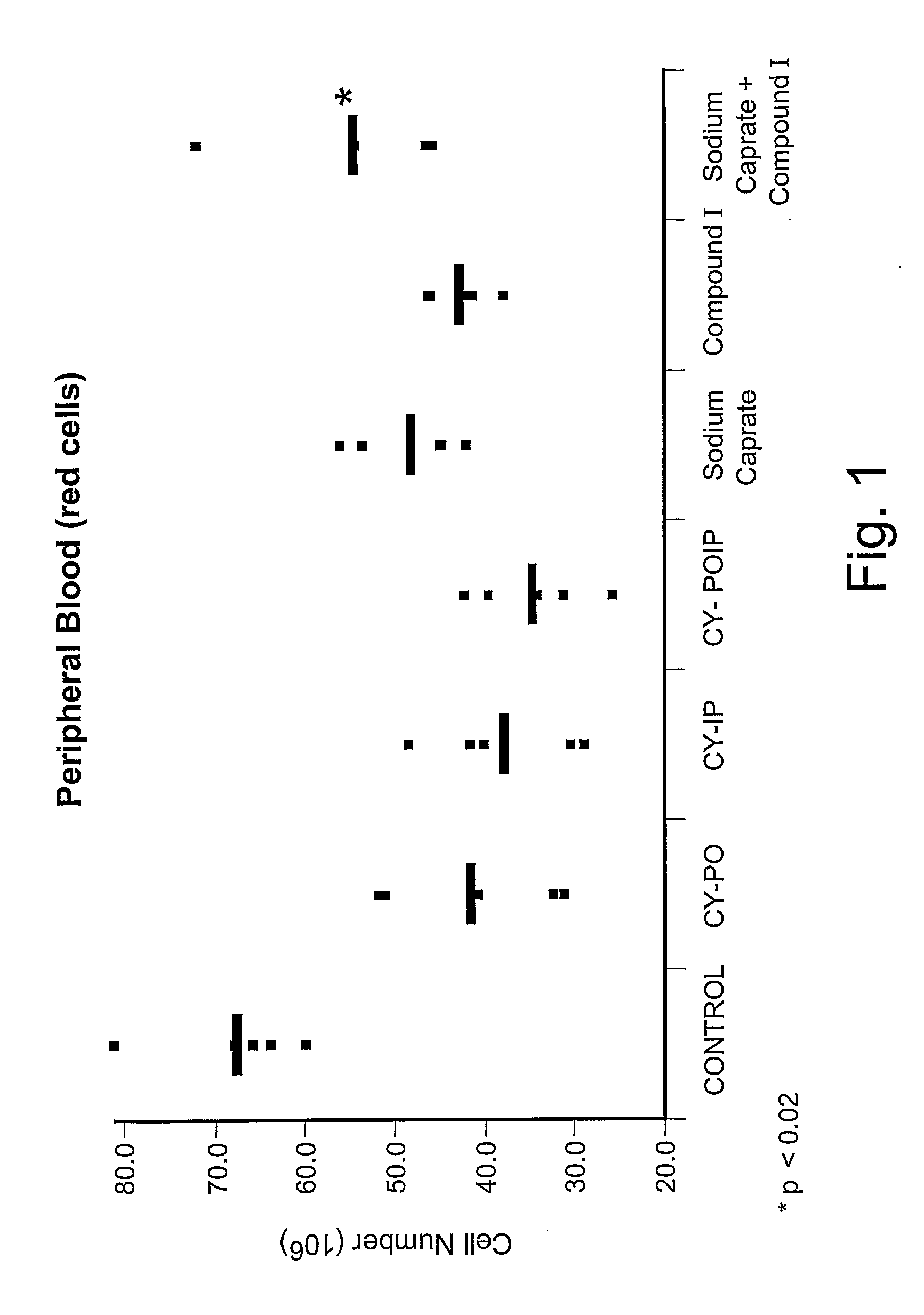
Substituted Purinyl Derivatives With Immunomodulator And Chemoprotective Activity And Use Alone Or With Medium-Chain Length Fatty Acids Or Glycerides
InactiveUS20080090848A1Enhanced chemoprotective activityHigh activityBiocideAntinoxious agentsTG - TriglycerideChemoprotective
The present invention describes new biological activities of immunomodulating 6-substituted purinyl compounds which make them particularly useful during the treatment of cancer. Collectively, these new biological activities make these purinyl compounds useful chemoprotective agents for the treatment of myelosuppression which is associated with cancer chemotherapy and / or radiotherapy. This chemoprotective activity is in addition to the immunomodulating and subsequent anticancer activity displayed by these compounds. The chemoprotective usefulness of these compounds is further enhanced by the use of medium-chain fatty acids or salts or triglycerides or mono- or diglycerides in combination with the 6-substituted purinyl compounds of this invention.
Owner:PROMETIC BIOSCIENCES LTD
Isoxazole derivative, preparation method therefor, and use thereof
ActiveUS20200339558A1Improved pharmacokinetic propertiesPromote activationOrganic active ingredientsOrganic chemistryDiseaseMetabolite
The present invention generally relates to an isoxazole derivative, a preparation therefor, and a use thereof. In particular, the present invention provides a farnesoid X receptor (FXR) agonist compound, and a stereoisomer, a tautomer, a polymorph, a solvate (e.g., a hydrate), a pharmaceutically acceptable salt, an ester, a metabolite, and an N-oxide, and the chemically protected forms and prodrugs thereof. The present invention further provides a preparation method for the compound, an intermediate thereof, and a pharmaceutical composition and kit containing the same and used thereof for treating FXR-mediated diseases or conditions.
Owner:SICHUAN KELUN BIOTECH BIOPHARMACEUTICAL CO LTD
3beta-hydroxy-ergosta-5-ene steroid derivative and drug application thereof
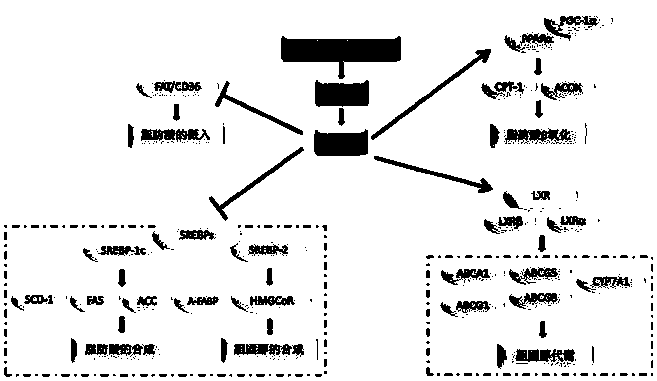
ActiveCN109280069AFat-lowering effect is goodMetabolism disorderSteroidsSecondary hyperlipidemiaLipid lowering
The invention provides a structure of a 3beta-hydroxy-ergosta-5-ene steroid compound, and applications of the compound and the pharmaceutically acceptable form of chemical protection or a prodrug in the preparation of drugs capable of preventing or treating LXRbeta related metabolic syndromes. The LXRbeta related metabolic syndromes include hyperlipidemia, atherosclerosis or hypertension. Throughthe discovery of the evaluation of lipid-lowering activity in animal bodies, the compound has obvious effects on lipid lowering, so that the compound has potentials of further developing into a novellipid-lowering drug.
Owner:MARINE BIOMEDICAL RES INST OF QINGDAO CO LTD +1
Carbamic acid compounds comprising an ester or ketone linkage as HDAC inhibitors
This invention pertains to certain carbamic acid compounds of the formula (I), which inhibit HDAC (histone deacetylase) activity: wherein: J is a linking functional group and is independently:—O —C(═O)— or —C(═O)—O — or —C(═O)—; Cy is a cyclyl group and is independently: C3-20carbocyclyl, C3-20heterocyclyl, or C5-20aryl; and is optionally substituted; Q1 is a cyclyl leader group, and is independently a divalent bidentate group obtained by removing two hydrogen atoms from a ring carbon atom of a saturated monocyclic hydrocarbon having from 4 to 7 ring atoms, or by removing two hydrogen atoms from a ring carbon atom of saturated monocyclic heterocyclic compound having from 4 to 7 ring atoms including 1 nitrogen ring atom or 1 oxygen ring atom; and is optionally substituted; Q2 is an acid leader group, and is independently: C1-8alkylene; and is optionally substituted; or: Q2 is an acid leader group, and is independently: C5-20arylene; C5-20arylene-C1-7alkylene; C1-7alkylene-C5-20arylene; or C1-7alkylene-C5-20aryleneC1-7alkylene; and is optionally substituted; and pharmaceutically acceptable salts, solvates, amides, esters, ethers, chemically protected forms, and prodrugs thereof. The present invention also pertains to pharmaceutical compositions comprising such compounds, and the use of such compounds and compositions, both in vitro and in vivo, to inhibit HDAC,and in the treatment of conditions mediated by HDAC, cancer, proliferative conditions, psoriasis, etc.
Owner:TOPOTARGET UK LTD
Pyridopyrimidine compound and application thereof
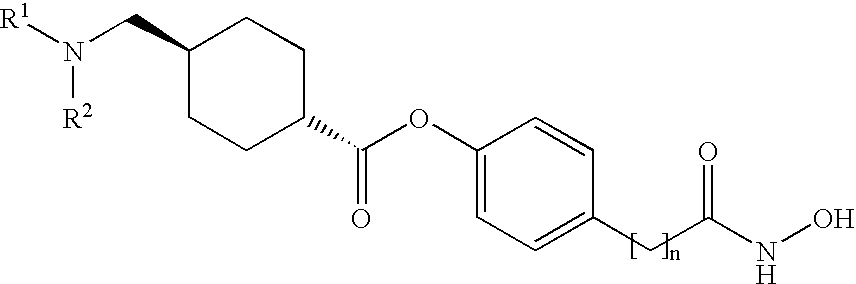
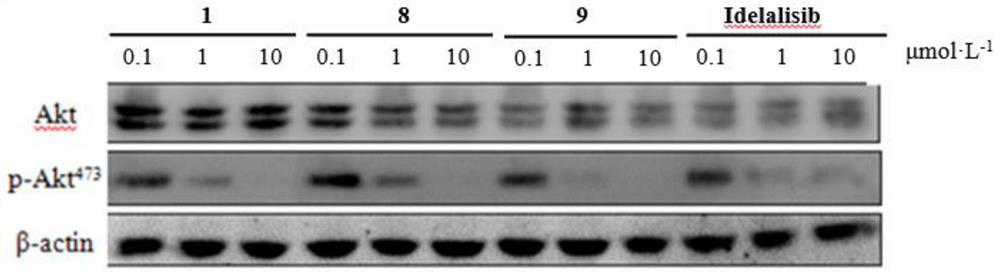
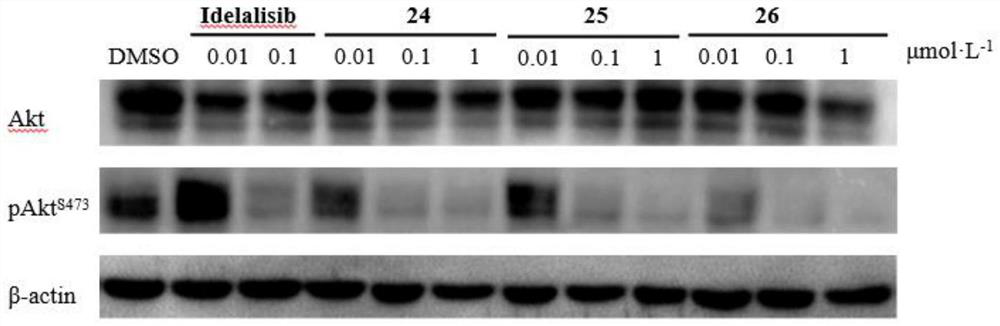
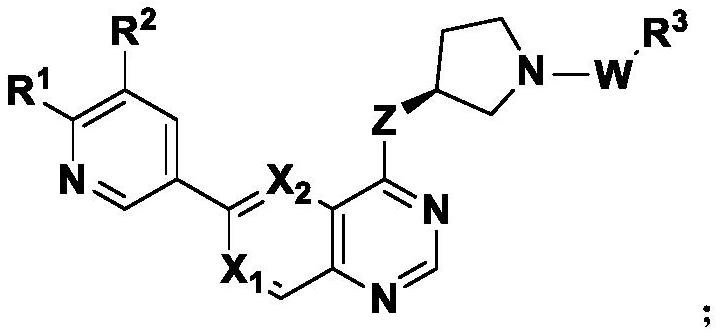
ActiveCN114315826ASelective activity is obviousEnhanced inhibitory effectOrganic chemistrySkeletal disorderDiseaseKinase activity
The invention discloses a pyridopyrimidine compound and application thereof, and belongs to the technical field of biological medicine. The pyridopyrimidine compound disclosed by the invention is a novel compound capable of being used as a PI3K delta inhibitor, and the determination on the activity of PI3K delta kinase and the selective activity of PI3K delta kinase proves that the compound disclosed by the invention has an obvious inhibiting effect on the activity of PI3K delta kinase and has an obvious selective effect on the activity of PI3K delta. In-vitro cell proliferation determination of a leukemia cell line shows that the compound disclosed by the invention has different inhibition effects on leukemia cells. The compounds disclosed by the invention, or pharmaceutically acceptable salts, deuterated compounds, hydrates, solvates, chemically protected prodrugs or combinations thereof, can be used as PI3K delta inhibitors, and play a role in treating and / or preventing inflammatory diseases or tumors.
Owner:XI AN JIAOTONG UNIV
Composition containing resveratrol and/or derivatives thereof and plant oil, process for producing said composition, nutraceutical and/or pharmaceutical product, and method for enhancing the potential of resveratrol
ActiveUS8912234B2Avoid rapid eliminationPromote absorptionCosmetic preparationsBiocideDiseaseAdditive ingredient
The present invention is a method for obtaining a formulation of resveratrol and rice bran oil. The resulting product in the form of an oil or solid proves to increase the therapeutic potential of resveratrol by the synergistic action of the components of the rice oil.The product obtained through the method of the invention is an active principle which, when incorporated in nutraceutical and / or pharmaceutical compositions, provides antioxidant, anti-inflammatory, antiviral, cardioprotective, neuroprotective and / or cancer chemoprotective action, besides protecting against infections and ischemia, reducing obesity, and preventing illnesses of old age.
Owner:UNIAO BRASILEIRA DE EDUCACAO E ASSISTENCIA MANTENEDORA DA PUC RS
Substituted purinyl derivatives with immunomodulator and chemoprotective activity and use alone or with medium-chain length fatty acids or glycerides
InactiveCN101080229AEnhanced chemoprotective activityAntinoxious agentsEster active ingredientsMyelosuppressive therapyChemoprotective
The present invention describes new biological activities of immunomodulating 6-substituted purinyl compounds which make them particularly useful during the treatment of cancer. Collectively, these new biological activities make these purinyl compounds useful chemoprotective agents for the treatment of myelosuppression which is associated with cancer chemotherapy and / or radiotherapy. This chemoprotective activity is in addition to the immunomodulating and subsequent anticancer activity displayed by these compounds, the chemoprotective usefulness of these compounds is further enhanced by the use of medium-chain fatty acids or salts or triglycerides or mono- or diglycerides in combination with the 6-substituted purinyl compounds of this invention.
Owner:PROMETIC BIOSCIENCES LTD
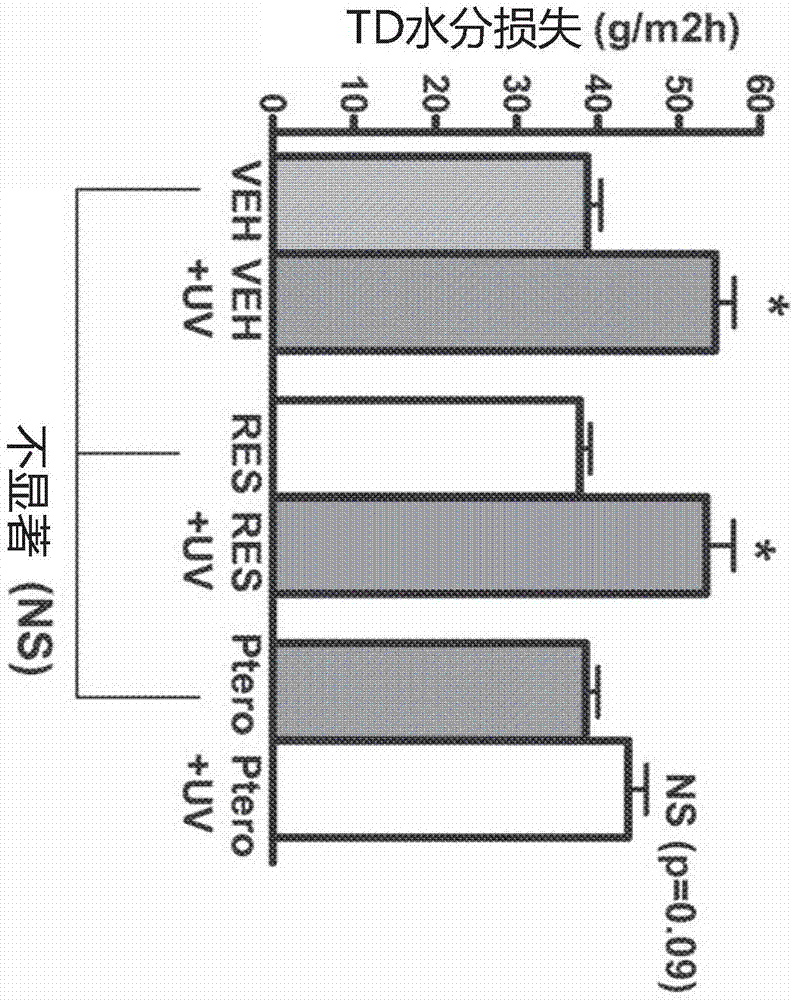
Topical pterostilbene compositions for use in treating UV-induced loss of barrier function in skin
InactiveCN107106877AImpaired inhibition or preventionPharmaceutical delivery mechanismEther/acetal active ingredientsSkin barrier functionChemoprotective
A chemoprotective method for treating, inhibiting or preventing loss of barrier function in skin caused by ultraviolet (UV) light by using an effective amount of pterostilbene is provided. Pharmaceutical and nutraceutical compositions containing pterostilbene suitable for administration to an individual in order to prevent subsequent UV-mediated loss of barrier function in skin are provided.
Owner:RGT UNIV OF CALIFORNIA
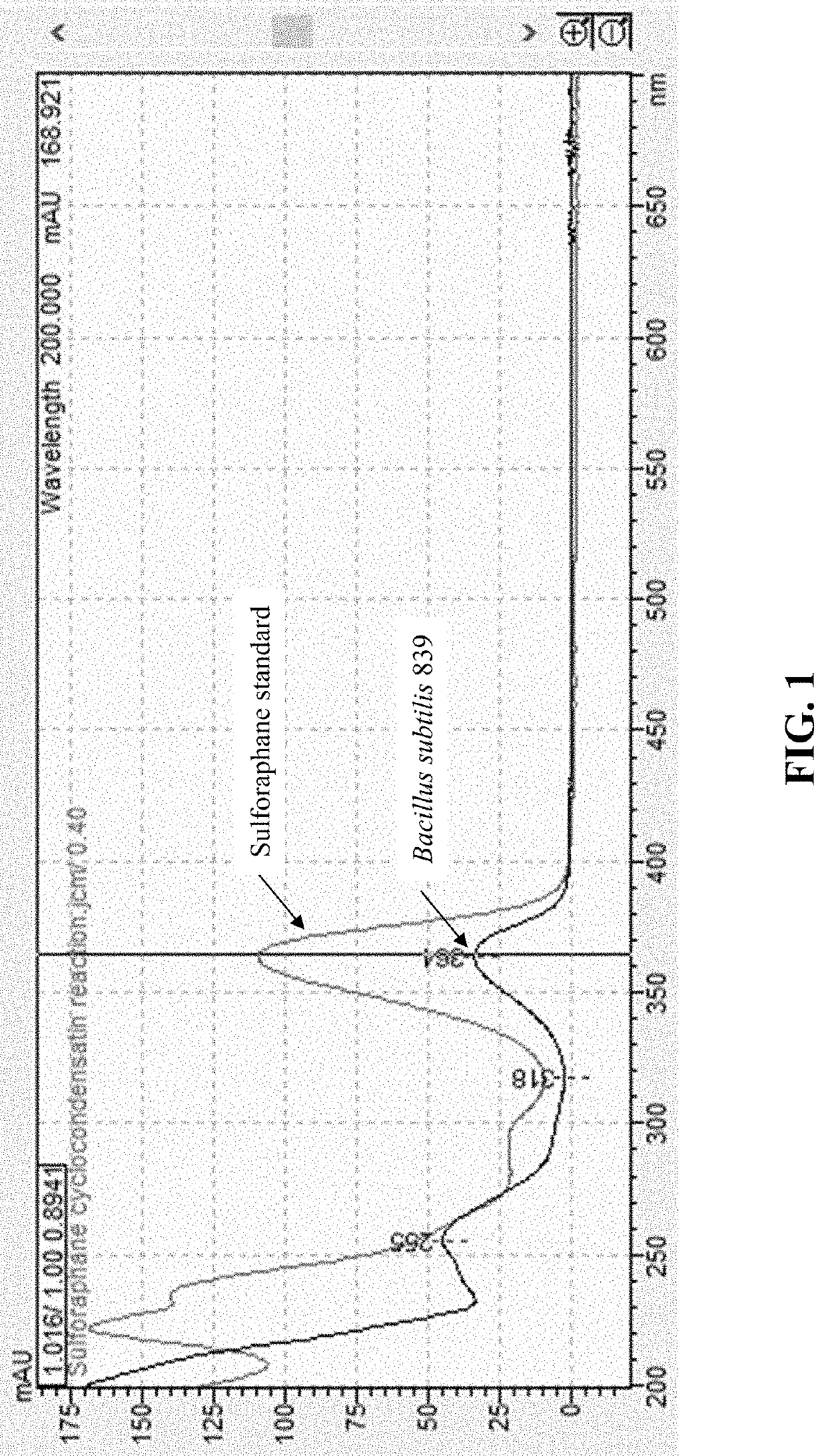
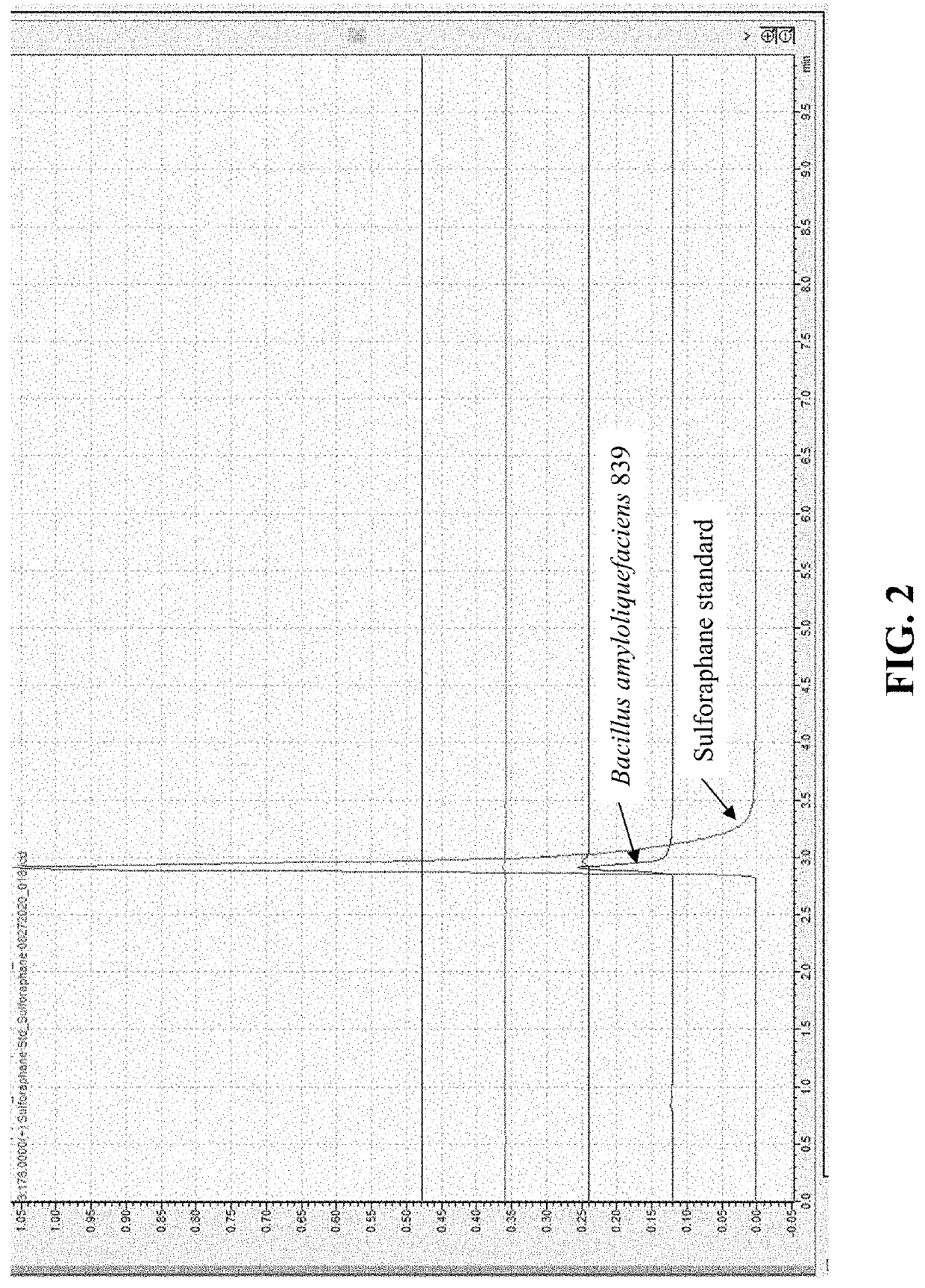
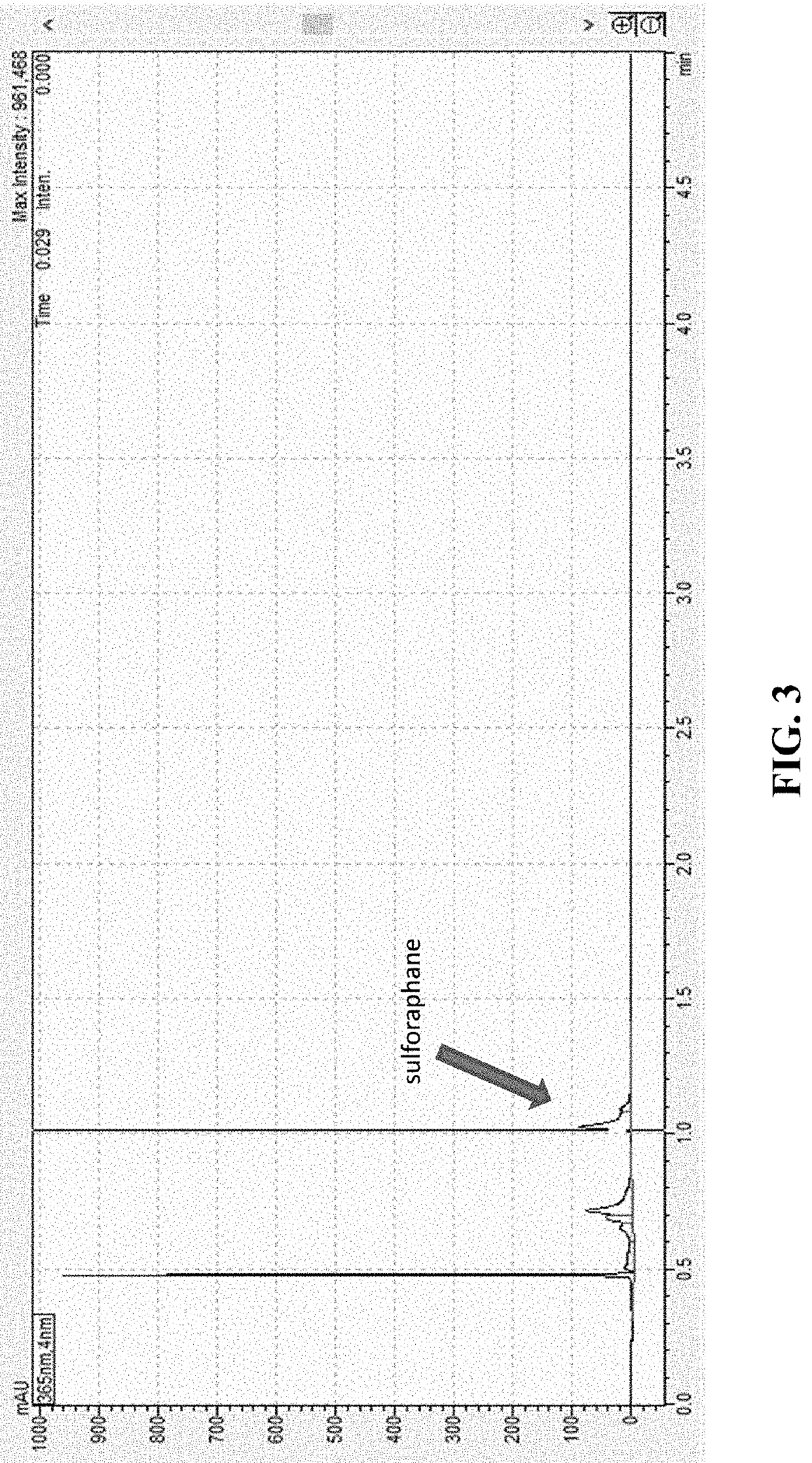
Compositions and methods to increase production of isothiocyanates
PendingUS20220193152A1Reduce inflammationIncreasing isothiocyanateBacteriaMicroorganism based processesBiotechnologyGlucoraphanin
Compositions and methods for converting at least one glucosinolate to an isothiocyanate using Bacillus subtilis 839, Bacillus subtilis CO4_4, Pediococcus pentosaceus M3_H01, and / or Pediococcus pentosaceus M2_H12, or active variants thereof, are provided. Conversion of glucosinolates, such as glucoraphanin, to isothiocyanates, such as sulforaphane, leads to the stimulation of the Nrf2 / Keap pathway and phase II enzymes, providing chemoprotective and anti-inflammatory effects. Accordingly, provided herein are compositions comprising Bacillus subtilis 839, Bacillus subtilis CO4_4, Pediococcus pentosaceus M3_H01, and / or Pediococcus pentosaceus M2_H12, or active variants thereof, for administration to subjects for increasing isothiocyanate (e.g., sulforaphane) production, increasing the expression of genes regulated by the Nrf2 transcription factor, including phase II enzymes, decreasing inflammation, and treating or preventing an inflammatory disorder or a cancer. The composition can comprise at least one glucosinolate or a plant, plant part or an extract thereof comprising glucosinolate(s).
Owner:CHURCH & DWIGHT CO INC
Xanthine derivatives, their preparation methods and uses
The present invention relates to a class of xanthine derivatives, pharmaceutically acceptable salts thereof, solvates of said derivatives, solvates of pharmaceutically acceptable salts, chemically protected forms or prodrugs thereof, and preparation methods thereof and Use; also relates to the intermediate compound used to prepare the xanthine derivative and the preparation method of the intermediate compound. The xanthine derivatives and pharmaceutical compositions thereof can effectively inhibit the activity of DPP-IV, and can be used to prepare medicines for diseases related to dipeptidyl peptidase (DPP-IV).
Owner:QILU PHARMA HAINAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds](https://images-eureka.patsnap.com/patent_img/160c93b4-7535-4186-a3b0-42b5846b0524/US20090325945A1-C00001.png)
![Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds](https://images-eureka.patsnap.com/patent_img/160c93b4-7535-4186-a3b0-42b5846b0524/US20090325945A1-C00002.png)
![Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds Imidazo[4, 5-b]pyridin-2-one and oxazolo[4, 5-b]pyridin-2-one compounds and analogs thereof as cancer therapeutic compounds](https://images-eureka.patsnap.com/patent_img/160c93b4-7535-4186-a3b0-42b5846b0524/US20090325945A1-C00003.png)
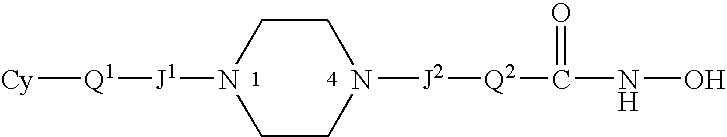
![Imidazo[4,5-b]pyridin-2-one and oxazolo[4,5-b]pyridin-2-one compounds and analogs thereof as therapeutic compounds Imidazo[4,5-b]pyridin-2-one and oxazolo[4,5-b]pyridin-2-one compounds and analogs thereof as therapeutic compounds](https://images-eureka.patsnap.com/patent_img/b0cd26c9-2c93-4765-aca7-10b49de51d24/US07625922-20091201-C00001.png)
![Imidazo[4,5-b]pyridin-2-one and oxazolo[4,5-b]pyridin-2-one compounds and analogs thereof as therapeutic compounds Imidazo[4,5-b]pyridin-2-one and oxazolo[4,5-b]pyridin-2-one compounds and analogs thereof as therapeutic compounds](https://images-eureka.patsnap.com/patent_img/b0cd26c9-2c93-4765-aca7-10b49de51d24/US07625922-20091201-C00002.png)
![Imidazo[4,5-b]pyridin-2-one and oxazolo[4,5-b]pyridin-2-one compounds and analogs thereof as therapeutic compounds Imidazo[4,5-b]pyridin-2-one and oxazolo[4,5-b]pyridin-2-one compounds and analogs thereof as therapeutic compounds](https://images-eureka.patsnap.com/patent_img/b0cd26c9-2c93-4765-aca7-10b49de51d24/US07625922-20091201-C00003.png)