Substituted purinyl derivatives with immunomodulator and chemoprotective activity and use alone or with medium-chain length fatty acids or glycerides
A technology of use and medicine, applied in the direction of organic active ingredients, medical preparations containing active ingredients, antidote, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
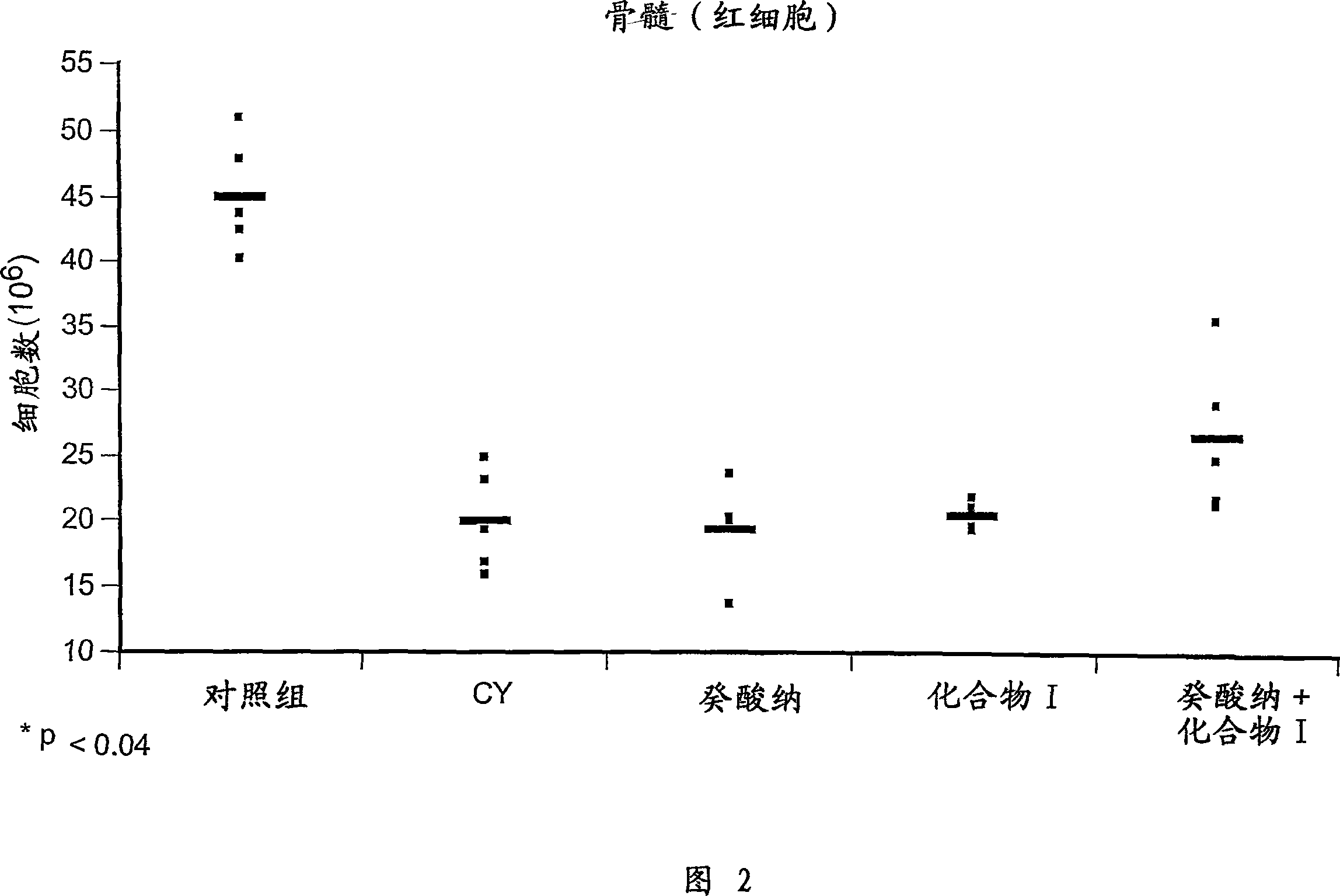
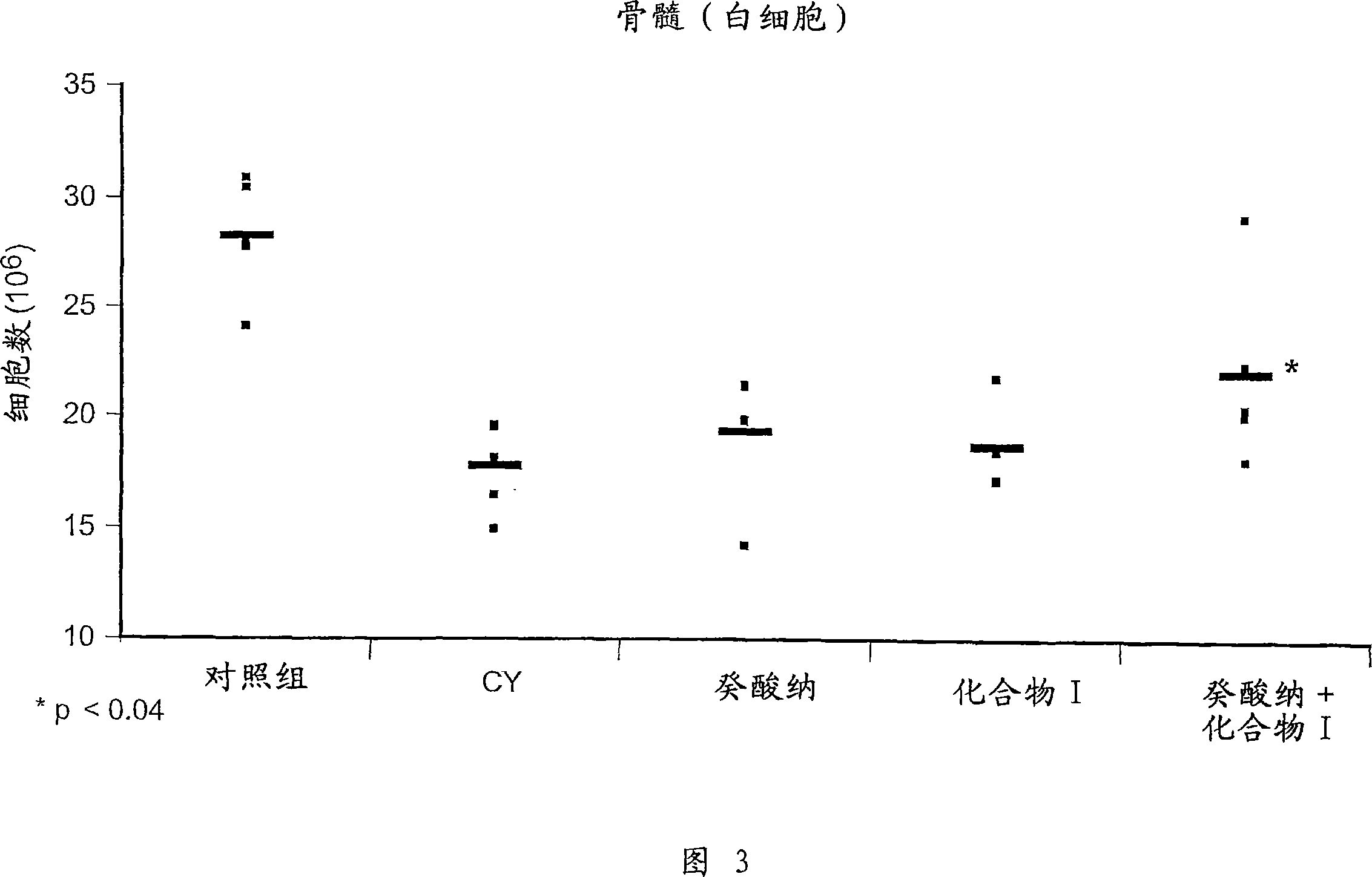
[0056] Example 1 Study on chemical protection: the protection of compound I on hematopoietic cells in vivo.
[0057] Female C57BL / 6 mice aged 6 to 8 weeks were treated with 200 mg / kg cyclophosphamide (CY), administered intravenously on day 0 to cause immunosuppression. To examine the chemoprotective effect of compound I, mice were previously injected intraperitoneally with 50 mg / kg of the compound on days -3, -2 and -1. Mice were sacrificed on day +5 by cardiac exsanguination and vertebral dissection. Afterwards, cells were obtained from thymus, spleen and bone marrow to prepare cell suspensions as follows.
[0058] Triturate the tissue in PBS buffer, contaminating red blood cells in ACK buffer (155mM NH 4 Cl, 12mM NaHCO 3 , 0.1 mM EDTA, pH 7.3) dissolved for five minutes. Cells were then harvested by centrifugation, washed three times in PBS, and resuspended in tissue culture medium. Count the cells with a counter.
[0059] After prior administration of Compound I to CY...
Embodiment 2
[0061] Example 2 Study on chemical protection: Combined use of sodium caprate and compound I induces proliferation or protection of immune cells in vivo.
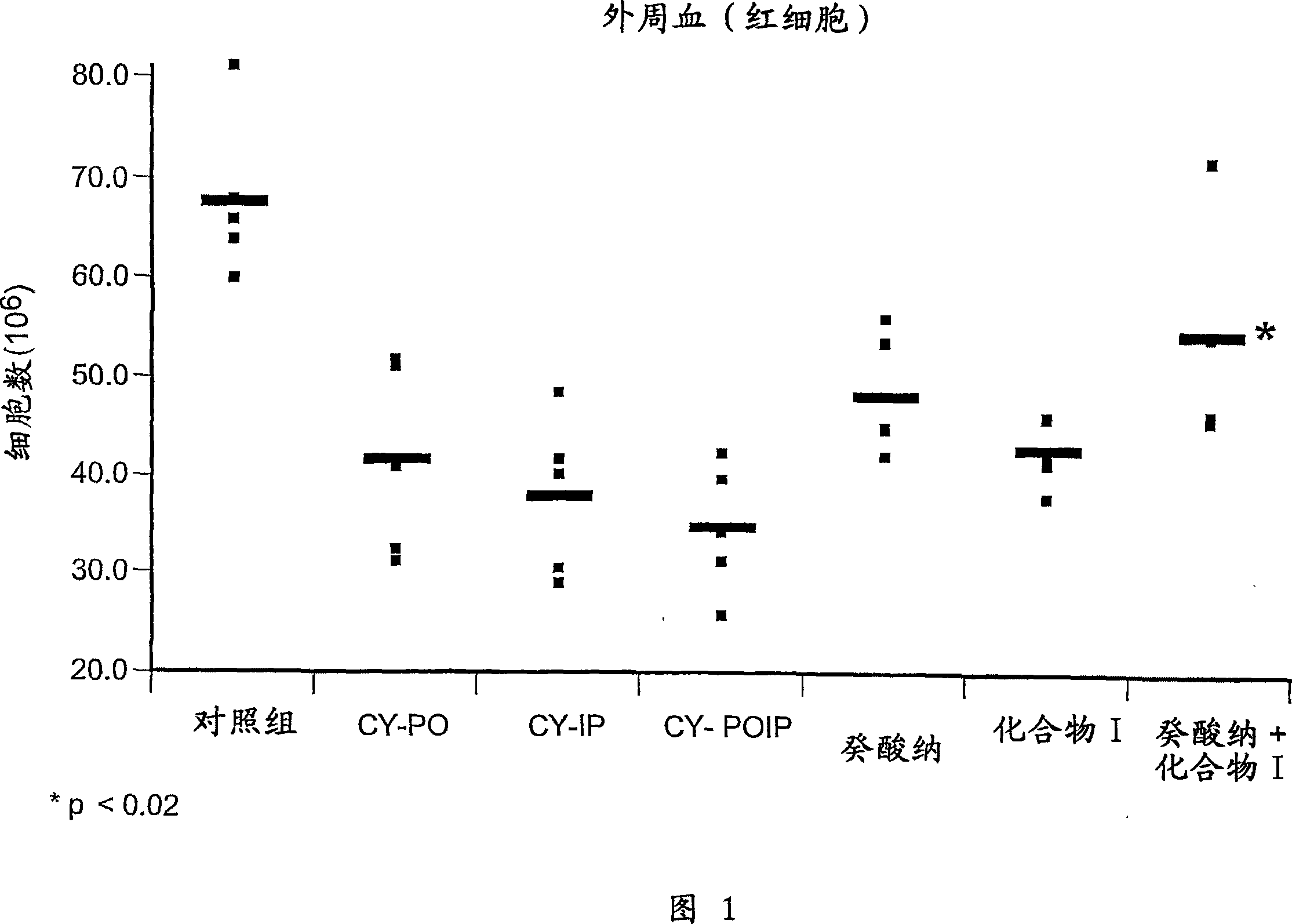
[0062] According to the protocol described in Example 1, the effect of sodium caprate, compound I, and the combination of the two compounds in inducing proliferation or protection of hematopoietic cells in vivo was determined. On days -3, -2 and -1, sodium caprate was administered orally (60.5 mM) and / or compound I was administered intraperitoneally (50 mg / kg). Treated animals compared to their respective control groups: CY+sodium caprate versus CY-PO (CY+PBS per well); CY+compound I versus CY-IP (CY+PBS intraperitoneal injection); CY+caprate Sodium + Compound I compared to CY-POIP (CY + PBS per well and PBS ip injection).
[0063] Accompanying drawing 1 shows the influence of sodium caprate, compound I and two kinds of compounds combination on peripheral red blood cell count. CY-treated mice pre-treated with sodium capra...
Embodiment 3
[0065] Example 3 Study on chemical protection: combined use of tricaprin and compound I induces proliferation or protection of immune cells in vivo.
[0066] According to the protocol described in Example 1, the effects of tricaprin, compound I and the combination of the two compounds in inducing proliferation or protection of hematopoietic cells in vivo were determined. Tricaprin (60.5 mM) was administered orally and / or compound I was injected intraperitoneally on days -3, -2 and -1.
[0067] Table 2 shows the effects of tricaprin, compound I and the combination of the two compounds on bone marrow erythrocyte count. A significant increase in bone marrow erythrocytes was obtained when CY-treated mice were previously treated with the combination of tricaprate and compound I. This is a synergistic effect compared with CY alone. Furthermore, the number of CFU-GEMM cells in bone marrow was significantly increased (3-fold) in mice treated with tricaprate and Compound I (Table 3)....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com