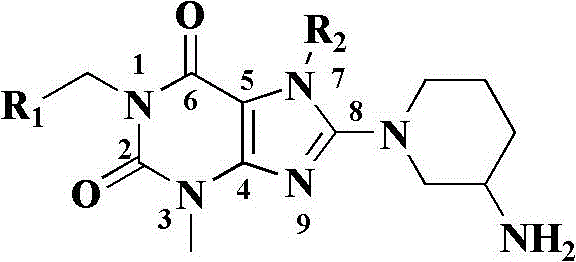
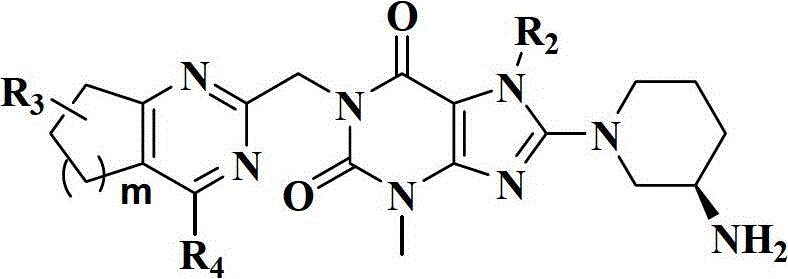
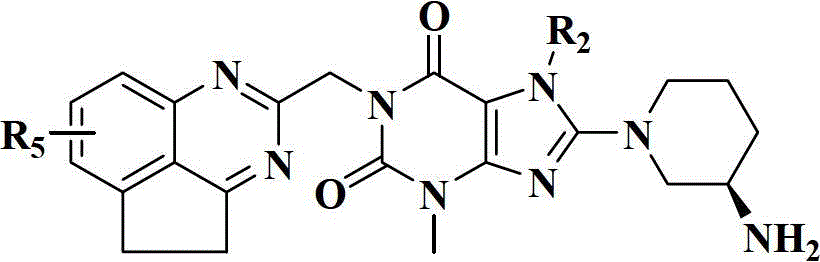
Xanthine derivatives, their preparation methods and uses
A technology of xanthine and derivatives, applied in the field of medicine, can solve the problems of weakening the activity of T cells and affecting the immune function of the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145] Example 1: 1-[(4,5-dihydrocyclopenta[de]quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)- Preparation of 8-(3-(R)-aminopiperidin-1-yl)xanthine (compound 1)
[0146] (1) Preparation of 3-methyl-7-(2-butyn-1-yl)-8-bromoxanthine
[0147]
[0148] At room temperature, 8-bromo-3-methyl-3,7-dihydro-purine-2,6-dione (24.5g, 100mmol) was suspended in 150mL N,N-dimethylformamide (abbreviated For: DMF), diisopropylethylamine (27mL, 150mmol) was added, mechanically stirred for 10 minutes, and then N,N-dimethyl Formamide solution (50 mL), after the dropwise addition, was stirred at room temperature for 10-12 hours. After the reaction was completed, the reaction solution was poured into ice water, stirred to precipitate a solid, filtered with suction, and dried in vacuo to obtain 25 g of a light yellow solid with a yield of 84.1%. ES-API(m / z):[M+H] + 297.0, 299.0.
[0149] (2) Preparation of 3-methyl-7-(2-butyn-1-yl)-8-[(R)-3-(tert-butoxycarbonylamino)piperidin-1-yl]xanthin...
Embodiment 2
[0174] Example 2: 1-[(4,5-dihydrocyclopenta[de]quinazolin-2-yl)methyl]-3-methyl-7-(3-methyl-2-butane Preparation of en-1-yl)-8-(3-(R)-aminopiperidin-1-yl)xanthine (compound 2)
[0175] (1) Preparation of 3-methyl-7-(3-methyl-2-buten-1-yl)-8-bromoxanthine
[0176]
[0177] At room temperature, 8-bromo-3-methyl-3,7-dihydro-purine-2,6-dione (24.5g, 100mmol) was suspended in 150mL N,N-dimethylformamide (abbreviated as: DMF), then added diisopropylethylamine (27mL, 150mmol), mechanically stirred for 10 minutes, then added dropwise 1-bromo-3-methyl-2-butene (16.5g, 110mmol) in N, 50 mL of N-dimethylformamide solution, after the dropwise addition, stirred at room temperature for 10-12 hours. After the reaction was completed, the reaction solution was poured into 300 ml of ice water to precipitate a solid, filtered by suction, and dried in vacuo to obtain 28.5 g of a light yellow solid, with a yield of 91.1%. ES-API(m / z):[M+H] + 313.0, 315.0.
[0178] (2) 3-methyl-7-(3-meth...
Embodiment 3
[0190] Example 3: 1-[(5,6,7,8-tetrahydro-4-methylquinazolin-2-yl)methyl]-3-methyl-7-(2-butyne-1- Base)-8-(3-(R)-aminopiperidin-1-yl)xanthine (compound 3)
[0191] (1) Preparation of 2-chloromethyl-5,6,7,8-tetrahydro-4-methylquinazoline (intermediate II(A)-1)
[0192]
[0193] Intermediate II(A)-1
[0194] 2-Acetylcyclohexanone (2.8g, 20mmol), potassium carbonate (4.1g, 30mmol) and chloroacetamidine hydrochloride (2.8g, 22mmol) were added to 30mL of n-butanol, and stirred under reflux for 5 hours. After the reaction was completed, the solvent was evaporated under reduced pressure, and the residue was poured into 50 mL of water, extracted with ethyl acetate (30 mL, extracted 3 times), the organic phases were combined, washed once with 30 mL of saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure A yellow solid was obtained, which was purified by column chromatography (eluent: petroleum ether / ethyl acetate=9:1) to obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com