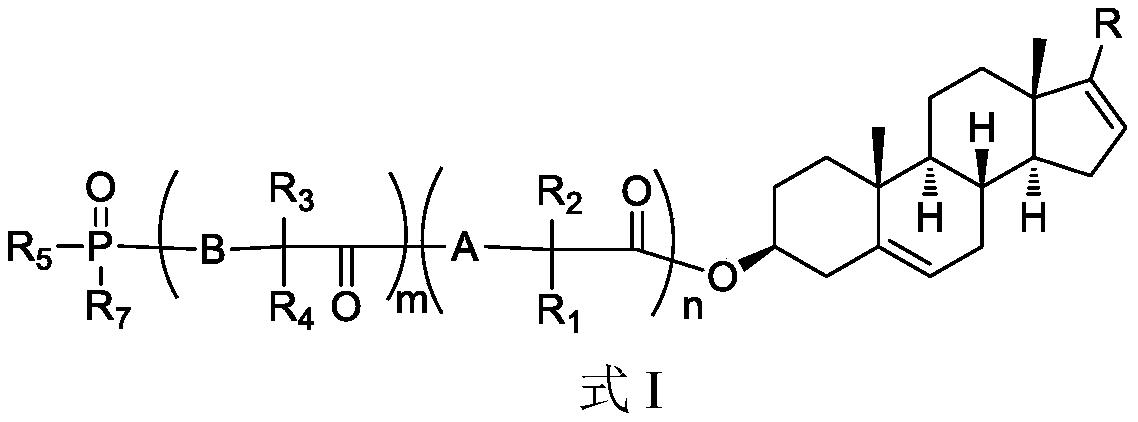
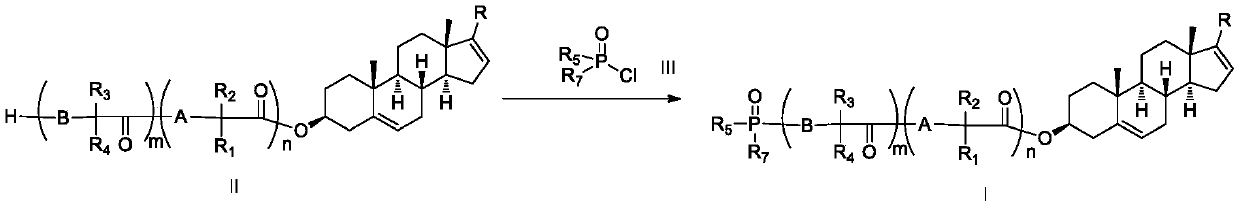
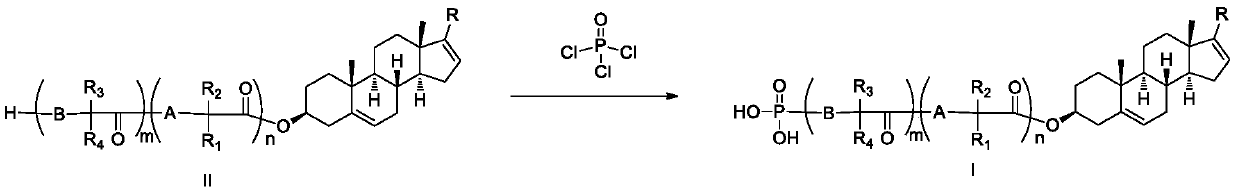
Phosphoryl-containing compounds and application thereof
A compound and acyl technology, applied in the field of preparation of phosphoryl-containing compounds, can solve the problems of high liver and heart toxicity and low bioavailability of abiraterone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0148] The synthesis of embodiment 1 compound I-1
[0149] Synthesized from 1 mmol of compound II-1 and 1.6 mmol of phosphorus oxychloride according to method B to obtain the title compound I-1 with a yield of 70%.
[0150] 1H NMR (500MHz, CDCl3), δ: 8.62(s, 1H, Py2-H), 8.45(d, J=5.5Hz, 1H, Py6-H), 7.66(d, J=10Hz, 1H, Py4-H ),7.65~7.53(m,4H),7.21~7.25(m,1H,Py5-H),6.00(s,1H,16-H),5.40(d,J=6Hz,1H,6-H), 3.51~3.58(m,1H),3.90(s,2H),3.66~3.42(m,1H,3α-H),2.62~2.59(m,2H),2.30~2.27(m,2H),2.20~2.13 (m,4H),2.11~2.06(m,2H),1.93~1.90(m,1H),1.89~1.77(m,3H),1.64~1.40(m,4H),1.07(s,3H,18- CH 3 ),1.05(s,3H,19-CH 3 ),0.94(d,J=5Hz,3H),0.87(d,J=10Hz,3H).
Embodiment 2
[0151] The synthesis of embodiment 2 compound I-2
[0152] Synthesized from 1 mmol of compound II-2 and 1.6 mmol of III-2 according to general method A to obtain the title target compound I-2 with a yield of 72%.
[0153] 1H NMR (500MHz, CDCl3), δ: 8.62(s, 1H, Py2-H), 8.42(d, J=5.5Hz, 1H, Py6-H), 7.74(d, J=9.5Hz, 1H, Py4- H), 7.18~7.14(m,1H,Py5-H),6.00(s,1H,16-H),5.40(d,J=6.5Hz,1H,6-H),5.18~5.01(m,2H ),4.66~4.61(m,1H,3α-H),3.88(s,2H),3.50(s,6H),3.47(d,J=4.5Hz,1H),2.59~2.27(m,2H), 2.26~2.19(m,2H),2.13~2.09(m,1H),1.94~1.91(m,2H),1.90~1.87(m,1H),1.86~1.62(m,4H),1.61~1.52(m ,4H),1.05(s,3H,18-CH 3 ),1.00(s,3H,19-CH 3 ),0.94(d,J=5.5Hz,3H),0.94~0.87(m,2H),0.87(d,J=9.5Hz,3H).
Embodiment 3
[0154] The synthesis of embodiment 3 compound Ⅰ-4
[0155] Synthesized from 1 mmol of compound II-4 and 1.6 mmol of phosphorus oxychloride according to method B to obtain the title compound I-4 with a yield of 65%.
[0156] 1H NMR (500MHz, CDCl3), δ: 7.84(s, 1H, 2'-H), 7.70~7.60(m, 2H, Ar-H), 7.26~7.22(m, 1H, Ar-H), 7.11~ 7.07(m,1H,Ar-H),5.84(s,1H,16-H),5.19(d,J=5.5Hz,1H,6-H),4.66~4.59(m,1H,3α-H) ,3.88(s,2H),3.52(s,6H),3.47(d,J=5Hz,1H),2.32~2.24(m,2H),2.13~2.11(m,1H),1.99~1.96(m, 2H),1.95~1.55(m,4H),1.78~1.57(m,2H),1.67~1.60(m,2H),1.60~1.57(m,3H),1.21~1.19(m,1H)1.05,1.11 ~1.05(m,3H),1.05(s,3H,18-CH 3 ),1.00(s,3H,19-CH 3 ),0.94(d,J=5Hz,3H),0.87(d,J=10Hz,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com