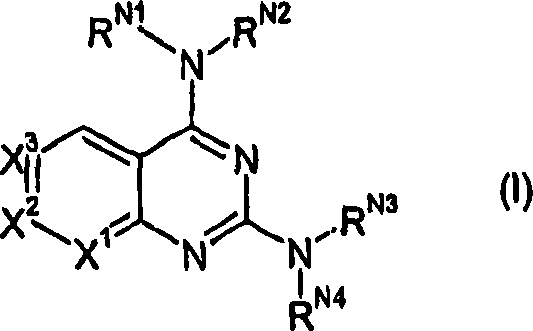
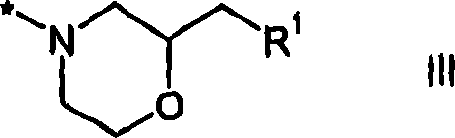
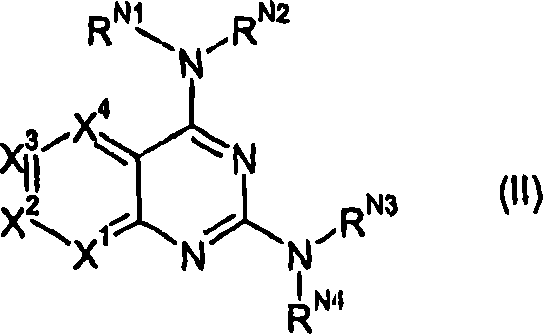
2,4-diamino-pyridopyrimidine derivatives and their use as mTOR inhibitors
A technology of uses and groups, applied in the field of mTOR inhibitor compounds, can solve problems such as signal increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0212]
[0213] raw material:
[0214] 1a: X 1 =N,X 2 =CH,X 3 =CH,X 4 =CH; 2-aminonicotinic acid
[0215] 1b:X 1 =CH,X 2 =CH,X 3 =N,X 4 =CH; 3-aminoisonicotinic acid
[0216] 1c:X 1 =CH,X 2 =CH,X 3 =CH,X 4 =N; 3-Amino-pyridine-2-carboxylic acid
[0217] (i) 1-H-pyridopyrimidine-2,4-dione (2)
[0218] Suspend the appropriate amino acid (1) (1 equivalent), potassium cyanate (5 equivalents) and ammonium chloride (10 equivalents) in water. The slurry was heated (160°C) and mixed by hand for 2 hours at which time water vapor was vented from the reactor. The reaction temperature was then raised to 200°C for 40 minutes, then cooled to 90°C while adding hot water, after which the mixture was allowed to cool to room temperature. The precipitate formed during cooling was removed by filtration, washed with water (twice) and diethyl ether (once) before drying in a desiccator to give the desired product of appropriate purity which was used without further purification. ...
Embodiment 2
[0235]
[0236] To 2-(2-chloromethyl-morpholin-4-yl)-4-((S)-3-methyl-morpholin-4-yl)-pyrido[2,3-d]pyrimidine (4aj ) (36mg, 0.1mmol) and a solution of the appropriate amine dissolved in dimethylacetamide (2.5ml) were added NaI (3mg, 0.02mmol) and K 2 CO 3 (14 mg, 0.1 mmol). The reactor was sealed and heated under the influence of microwave radiation (low absorption setting, 200°C, 10 minutes). The crude reaction mixture was then concentrated in vacuo and purified by preparative HPLC to give the desired product (5).
[0237]
[0238]
[0239]
[0240]
[0241] To 2-(2-chloromethyl-morpholin-4-yl)-4-((S)-3-methyl-morpholin-4-yl)-pyrido[2,3-d]pyrimidine (4aj ) (36mg, 0.1mmol) (11mg, 0.03mmol) in anhydrous dimethylacetamide (0.5ml) was added tert-BuOK (6.8mg, 06mmol) and 18-crown-6-ether (0.006mmol, 1.6 mg). The appropriate alcohol was then added to the reaction mixture and the reactions were heated to 110°C for 15 hours. The crude reaction mixture was then con...
Embodiment 3
[0244] Embodiment 3: biological test
[0245] For mTOR enzymatic activity assays, mTOR protein was isolated from HeLa cell cytoplasm by immunoprecipitation and activity was determined essentially as previously described (ref. 21 ) using recombinant cell PHAS-1 as substrate.
[0246] All tested compounds showed ICs less than 15 μM 50 value.
[0247] The following compounds exhibited ICs less than 1.5 μM 50 Values: 4c, 4d, 4h, 4n, 4o, 4y, 4ab, 4af, 4ag, 4ah, 5e, 5g, 5h, 5k, 5m, 5z.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com