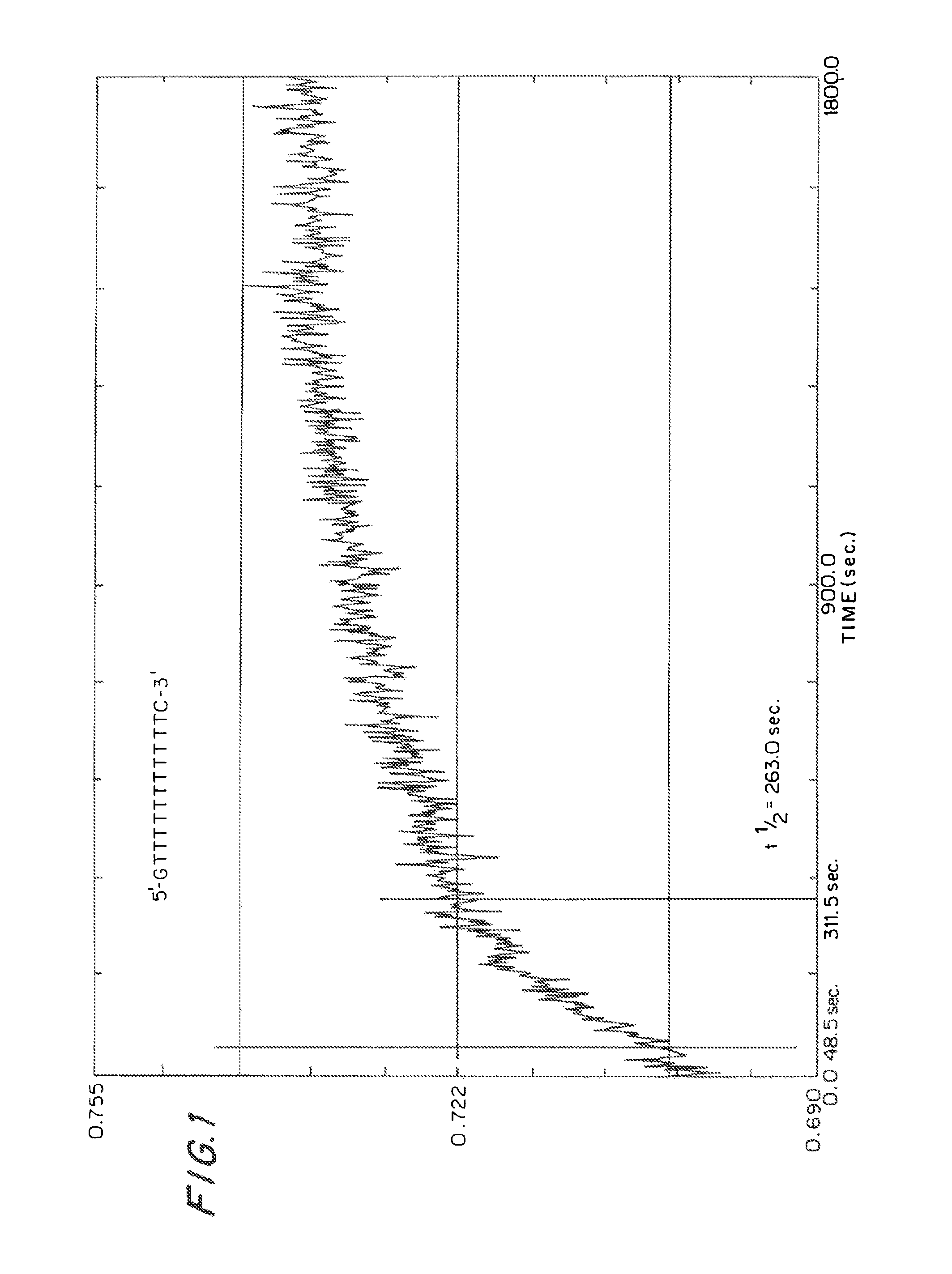
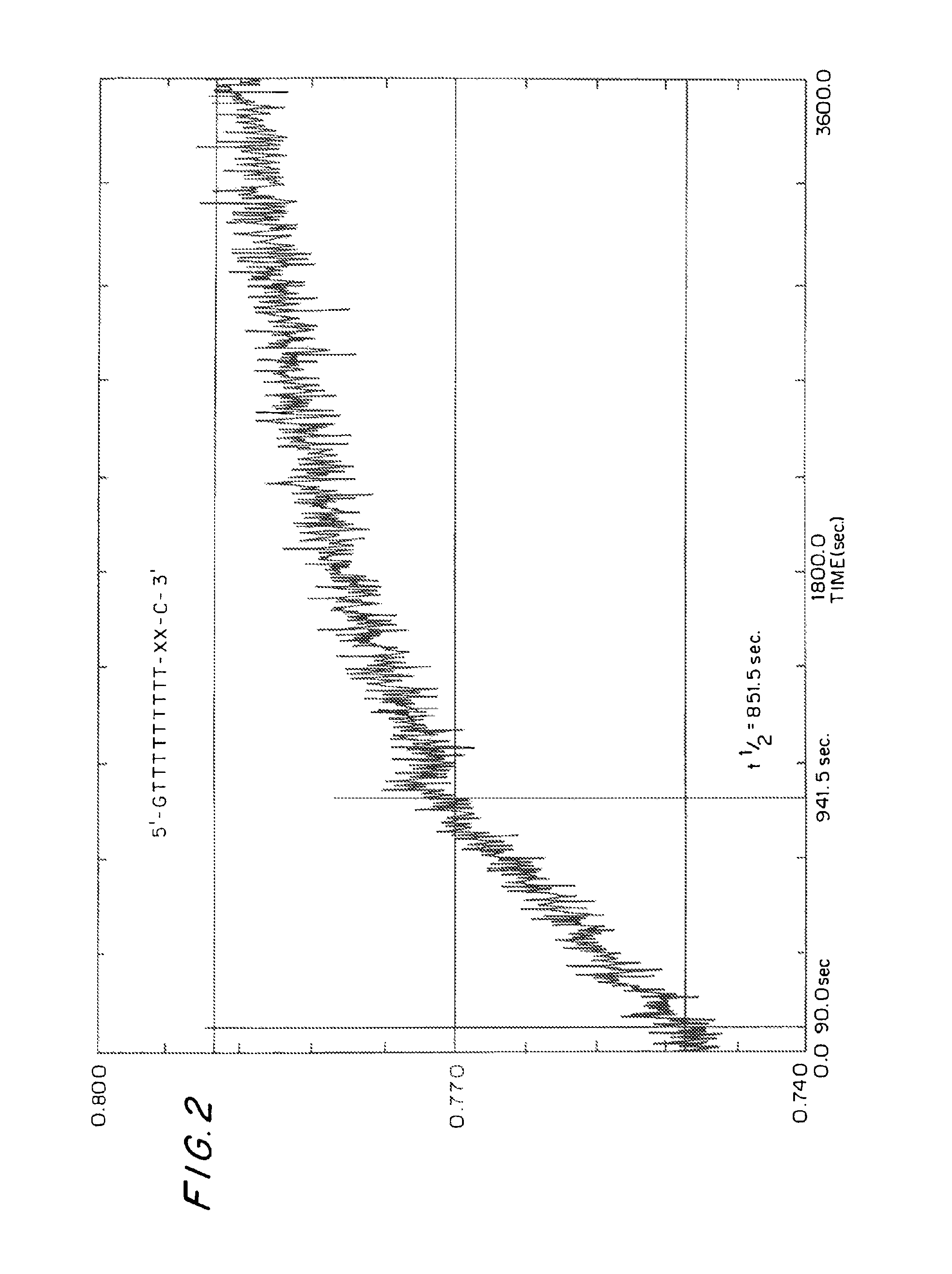
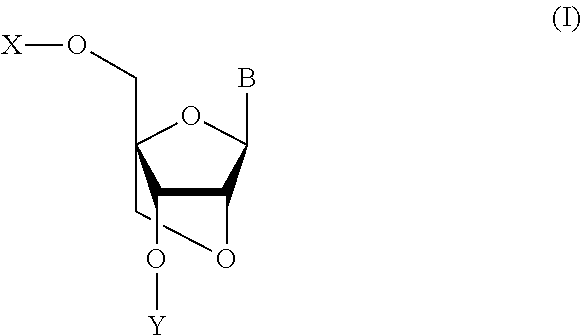
Bicyclonucleoside and oligonucleotide analogues
a technology of oligonucleotide and cyclonucleoside, which is applied in the field of new nucleotide analogues and novel nucleotide analogues, can solve the problems of insufficient in vivo stability of derivatives or analogues, insufficient sequence specificity, and inability to fully satisfy the requirements of in vivo stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Synthesis of Nucleoside Analogue
[0080](1) Synthesis of Methyl=5-O-(t-butyldiphenylsilyl)-4-hydroxymethyl-2,3-O-isopropylidene-β-D-ribofuranoside (Compound 14)
[0081]In a stream of nitrogen, Et3N (2.62 ml, 18.8 mmols) and t-butyldiphenylsilyl chloride (4.88 ml, 18.8 mmols) were added to an anhydrous CH2Cl2 solution (40 ml) of Compound 13 (2.00 g, 8.54 mmols) known in the literature under cooling with ice, and the mixture was stirred for 13 hours at room temperature. To the reaction mixture, a saturated sodium bicarbonate solution was added, whereafter the reaction system was extracted with AcOEt 3 times. The organic phase was washed once with a saturated sodium chloride solution, and then dried over anhydrous Na2SO4. The solvents were distilled off under reduced pressure, and the resulting crude product was purified by silica gel column chromatography (hexane:AcOEt=5:1) to obtain colorless oily matter (Compound 14) (2.82 g, 5.98 mmols, 70%).
[0082][α]D17−16.2° (c=0.52, CHCl3). IR ν (KB...
example 3
Synthesis of Nucleoside Analogue (Different Method)
[0115](1) Synthesis of 3-O-benzyl-5-O-t-butyldiphenylsilyl-4-(hydroxymethyl)-1,2-O-isopropylidene-α-D -erythropentofuranose (Compound 32)
[0116]In a stream of nitrogen, triethylamine (3.71 ml, 26.6 mmols) and t-butyldiphenylsilyl chloride (6.94 ml, 26.7 mmols) were added, under cooling with ice, to a methylene chloride solution (50 ml) of Compound 31 (2.50 g, 8.08 mmols) prepared in accordance with the aforementioned reference 5). The mixture was stirred for 10.5 hours at room temperature. After a saturated sodium bicarbonate solution was added to the reaction mixture, the system was extracted with ethyl acetate. The organic phase was washed with a saturated sodium chloride solution, and then dried over sodium sulfate. The solvents were distilled off under reduced pressure, and the resulting crude product was purified by silica gel column chromatography (AcOEt-hexane:=1:4→4:3) to obtain a white solid, Compound 32 (2.97 g, 5.41 mmols,...
example 4
[0157](1) Synthesis of 2′-O-acetyl-3′-O-benzyl-5′-O-t-butyldiphenylsilyl-4′-p-toluenesulfonyloxymethyl-N6-benzoyladenosine (Compound 40)
[0158]In a stream of nitrogen, a 1,2-dichloroethane solution (5.0 ml) of Compound 34 (250 mg, 0.336 mmol) and trimethylsilyltrifluoromethane sulfonate (6.7 μl, 0.0336 mmols) were added, at room temperature, to 2TMS.ABz (128.7 mg, 0.336 mmol) prepared In accordance with a reference 6) (H. Vorbrggen, K. Krolikiewicz and B. Bennua, Chem., Ber., 114, 1234-1255 (1981)). The mixture was heated under reflux for 26 hours. After a saturated sodium bicarbonate solution was added to the reaction mixture, the system was extracted 3 times with methylene chloride. The organic phase was washed with a saturated sodium chloride solution, and then dried over sodium sulfate. The solvents were distilled off under reduced pressure, and the resulting crude product was purified by silica gel column chromatography (CHCl3MeOH, 1:3) to obtain a white powder, Compound 40 (234...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting temperature | aaaaa | aaaaa |
| melting temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com