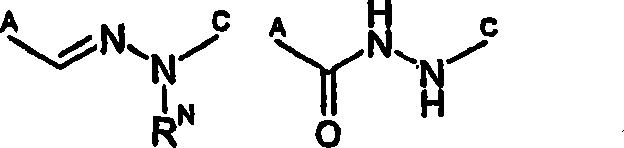
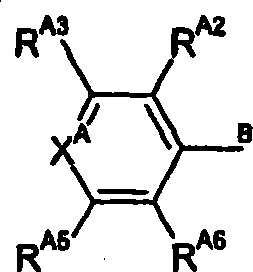
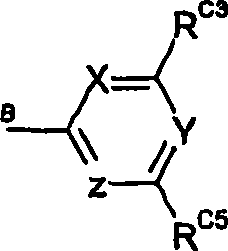
Hydrazinomethyl, hydr zonomethyl and 5-membered heterocyclic compounds which act as mTOR inhibitors and their use as anti cancer agents
A technology of compounds and solvates, applied in the field of mTOR inhibitor compounds, can solve problems such as signal increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a
[0317] Example 1a: 6-(N'-methylene-hydrazino)-[1,3,5]triazine-2,4-diamine derivative (5) preparation of
[0318]
[0319] (i) Preparation of 4,6-dichloro-[1,3,5]triazin-2-ylamine derivative (2)
[0320] To a cooled (-60°C) solution of cyanuric chloride (1) (3.00g, 16.26mmol) in ethylene glycol dimethyl ether (40ml) was added the appropriate amine (2.80ml, 32.5mmol) dissolved in water (1.4 ml) solution. The amine solution was added dropwise over 10 minutes. The mixture was removed from the cold water bath and water (25ml) was added to completely quench the reaction. The quench mixture was stirred for 5 minutes before any precipitate was filtered off. The filter cake was washed with water (250ml) and dried in a vacuum desiccator to give the desired 4,6-dichloro-[1,3,5]triazin-2-ylamine, which was then further purified by making Recrystallization from a minimal amount of hot EtOAc afforded the product.
[0321]
[0322] (iia) Synthesis of 6-chloro-[1,3,5]triazine-2...
Embodiment 1
[0338] Example 1(b): 4-[(4-chloro-6-morpholin-4-yl-[1,3,5]triazin-2-yl)-hydrazonomethyl]-2,6- Synthesis of Dimethoxy-phenol (7)
[0339]
[0340] (i) (4-chloro-6-morpholin-4-yl-[1,3,5]triazin-2-yl)-hydrazine (6)
[0341] Using the method of Example 1a(iii), the compound was synthesized from (2a) with a yield of 99%.
[0342] M / Z (LC-MS, ESP): 231 [M+H] + , R / T = 2.93 minutes.
[0343] (ii) 4-[(4 chloro-6-morpholin-4-yl-[1,3,5]triazin-2-yl)-hydrazonomethyl]-2,6-dimethoxy- Phenol(7)
[0344] This compound was synthesized from (6) using the method of Example 1a(iv).
[0345] M / Z (LC-MS, ESP): 97% purity, 395 [M+H] + , R / T = 4.08 minutes.
Embodiment 2
[0346] Example 2: Synthesis of 6-(N'-methylene-hydrazino)-pyrimidine-2,4-diamine derivatives (12)
[0347]
[0348] (i) Synthesis of 2,6-dichloro-pyrimidin-4-ylamine derivative (9)
[0349] To a cooled (-5 °C) solution of 2,4,6-trichloro-pyrimidine (8) (2.73 mmol) in ethanol (6 ml) was added the appropriate amine (2.73 mmol), followed by dropwise addition of Et 3 N (0.303ml, 2.18mmol). The cooling bath was removed and the reaction was allowed to warm to room temperature. Water was then added to the mixture, resulting in the formation of a precipitate. The solid was removed by suction filtration and washed with icy EtOH (6ml) to give the desired product which was then purified by flash chromatography (eluent typically 100% hexane to 4:1-hexane:EtOAc)
[0350]
[0351] (iia) Synthesis of 6-chloro-pyrimidine-2,4-diamine derivatives (10) (wherein the amine groups are different)
[0352] To a solution of the appropriate 2,6-dichloro-pyrimidin-4-ylamine derivative (9) (0.8...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com