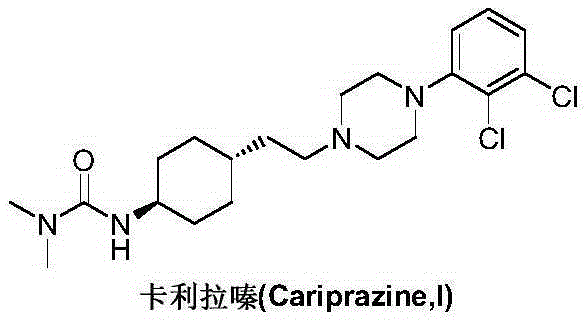
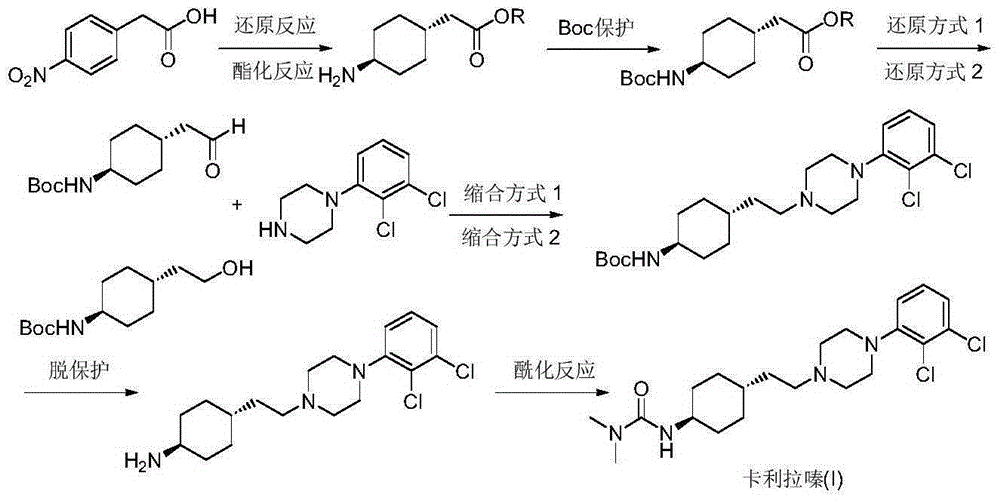
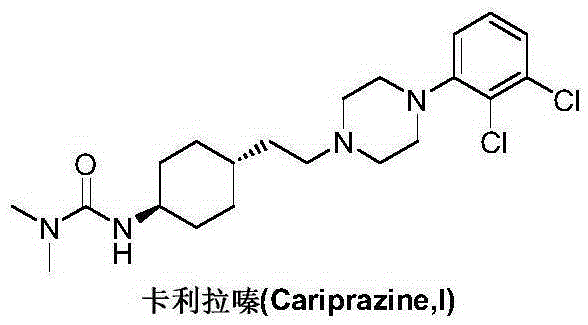
Preparation method of cariprazine
A technology for preparing cariprazine and its preparation steps, which is applied in the field of preparation of cariprazine for treating psychosis, and can solve problems such as cumbersome steps, harsh reaction conditions, high temperature and pressure, and achieve environmentally friendly and economical process and easy-to-obtain raw materials , the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 4-(2-hydroxyethyl)cyclohexanone (II) (1.42g, 10mmol), 1-(2,3-dichlorophenyl)piperazine (III) (2.30g, 10mmol) into the reaction flask , triphenylphosphine (3.14g, 12mmol) dry tetrahydrofuran 25mL, add dropwise a solution of diethyl azodicarboxylate (2.09g, 12mmol) in tetrahydrofuran 25mL under ice bath, dropwise, rise to room temperature, stir the reaction 3- After 4 hours, TLC detected that the reaction was complete. Distilled under reduced pressure, the residue was dissolved with ethyl acetate and n-hexane, the insoluble matter was filtered off, the filtrate was washed with water three times, and dried over anhydrous sodium sulfate. Concentrate, and recrystallize the crude product from n-hexane / ethyl acetate (2:1) to obtain off-white solid 4-[2-[4-(2,3-dichlorophenyl)piperazin)-1-yl]ethyl] Cyclohexanone (IV) 3.20g, yield 90.4%; 1 HNMR (CDCl 3 )δ7.19-7.21(m,2H),6.94-7.06(m,1H),3.63-3.67(m,2H),3.42-3.46(m,2H),3.01-3.30(m,4H),2.55- 2.58(m,4H),2.36-2..40(m,2H),1.85...
Embodiment 2
[0034] Add 4-[2-[4-(2,3-dichlorophenyl)piperazine)-1-yl]ethyl]cyclohexanone (IV) (1.77g, 5mmol), benzylamine ( 0.54g, 5mmol), 0.5g of 4A molecular sieve and 50mL of methanol, stirred at room temperature for 5-7 hours. After filtering, the filtrate was transferred to a hydrogenation reactor, 0.18 g of 10% palladium carbon was added, hydrogen gas was introduced, the pressure was kept at 2 atmospheres and the temperature was 35-40° C., and the reaction was carried out for 5-6 hours. TLC detects that the reaction is complete. Cool down to room temperature and recover the catalyst by filtration. Concentration and concentration under reduced pressure, the residue was slurried with n-hexane to obtain a yellow waxy solid trans-4-[2-[4-(2,3-dichlorophenyl)piperazin)-1-yl]ethyl]cyclo Hexylamine (V) 1.5g, yield 84.5%, 1 HNMR (CDCl 3 )δ7.11-7.20(m,2H),6.94-7.09(m,1H),3.09(m,4H),2.58-2.76(m,5H),2.38-2.49(m,2H),1.83-1.94( m,2H),1.74-1.82(m,2H),1.35-1.52(m,4H),1.18-1.32(m,1H),0.95-1.16(...
Embodiment 3
[0036]Add 4-[2-[4-(2,3-dichlorophenyl)piperazine)-1-yl]ethyl]cyclohexanone (IV) (1.77g, 5mmol), hydroxylamine hydrochloride ( 0.42g, 6mmol), sodium acetate (0.82g, 10mmol), 25mL each of ethanol and water, and stirred at room temperature for 12 hours. Concentrate, extract with dichloromethane, and dry over anhydrous sodium sulfate. The solvent was recovered under reduced pressure, 20 mL of acetic acid was added to the obtained solid, and zinc powder (2.6 g, 40 mmol) was added in portions at room temperature. The temperature was raised to 80-90° C., and the reaction was incubated for 2-3 hours. TLC detected that the reaction was complete. Filtrate, concentrate the filtrate under reduced pressure, adjust the residue to be alkaline with 10% sodium hydroxide solution, extract 3 times with ethyl acetate, combine the organic phases, concentrate under reduced pressure, and beat with n-hexane to obtain a yellow waxy solid trans-4 -[2-[4-(2,3-Dichlorophenyl)piperazin)-1-yl]ethyl]cyclo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com