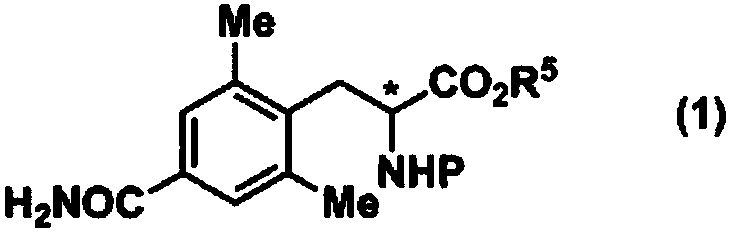
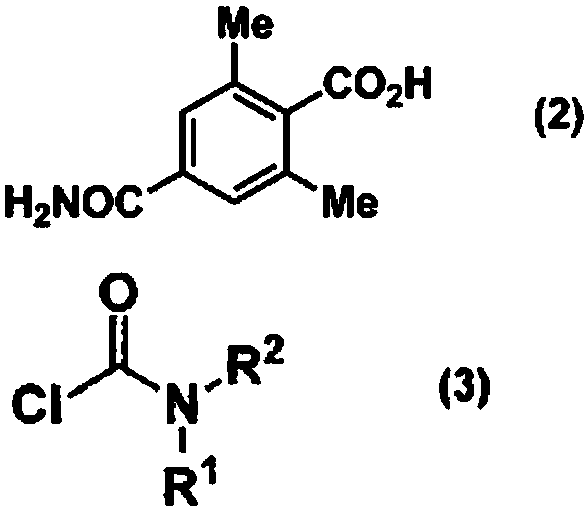
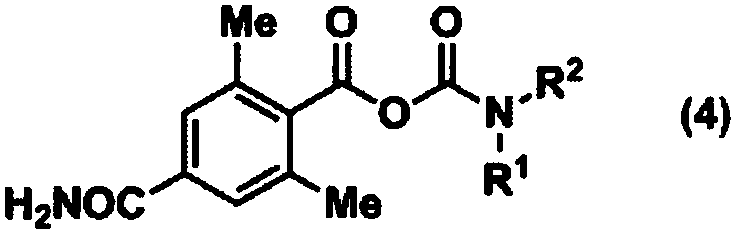
Method for producing optically active 4-carbamoyl-2,6-dimethylphenylalanine derivative
A technology of dimethylphenylalanine and carbamoyl, which is applied in the field of manufacture of optically active 4-carbamoyl-2,6-dimethylphenylalanine derivatives and can solve the problem of high environmental burden , high toxicity, and difficulty in industrial-scale implementation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0232] Hereinafter, examples are shown and the present invention will be described in more detail, but these examples do not limit the present invention at all.
reference example 1
[0233] (Reference Example 1) Production of 4-carbamoyl-2,6-dimethylbenzoic acid
[0234] A solution consisting of 2,4,6-trimethylbenzoic acid (500 g, 3.05 mol) and water (6500 mL) was cooled to 5 to 10° C., and sodium hydroxide (793 g, 19.83 mol) was added thereto. Next, potassium permanganate (1300 g, 8.23 mol) was added in portions over 12 hours. After stirring at 5-8 degreeC for 16 hours, sodium sulfite (20g) was added to the reaction liquid, and it stirred at 15-20 degreeC for 1 hour. The precipitated manganese dioxide was filtered off and washed with a 5% aqueous sodium hydroxide solution (1000 mL). Concentrated hydrochloric acid (approximately 600 mL) was added to the filtrate to make it acidic, whereby a solid was deposited, and the filtrate was stirred at 10° C. for 5 hours. The solid was filtered off under reduced pressure, washed with water (3000 mL), and the wet crystals were transferred to another container. Methanol (2500 mL) and water (2500 mL) were added th...
Embodiment 1
[0237] (Example 1) Production of 4-carbamoyl-2,6-dimethylbenzoic acid N,N-diethylcarbamic anhydride
[0238] To the N,N-dimethylformamide solution (1275 mL) of the compound (255 g, 1.32 mol) obtained in Reference Example 1, diethylcarbamoyl chloride ( 270g, 1.98mol), triethylamine (213g, 2.11mol), pyridine (104g, 1.32mol). After stirring for 16 hours, the reaction solution was cooled to 0-10° C., and water (3825 mL) was added dropwise while maintaining the temperature. While maintaining the temperature, the obtained slurry solution was stirred for 2 to 3 hours, and the precipitated solid was collected by filtration. The obtained solid was washed with water (500 mL), and dried under reduced pressure to obtain 339 g of the title compound (yield: 97%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com