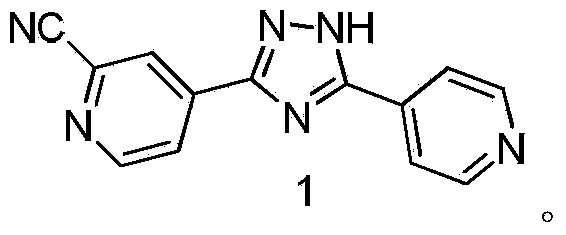
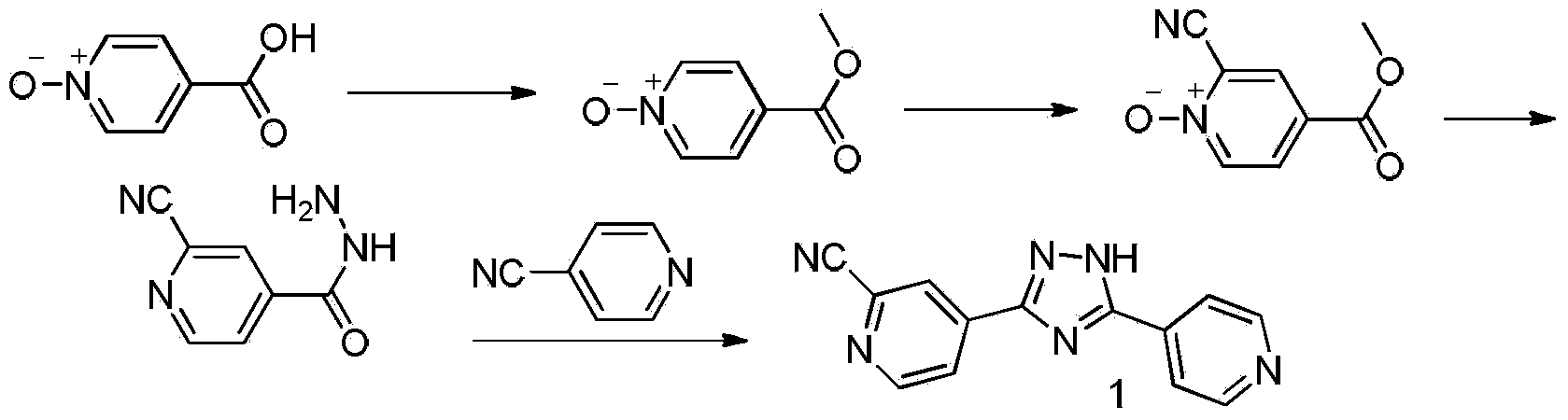
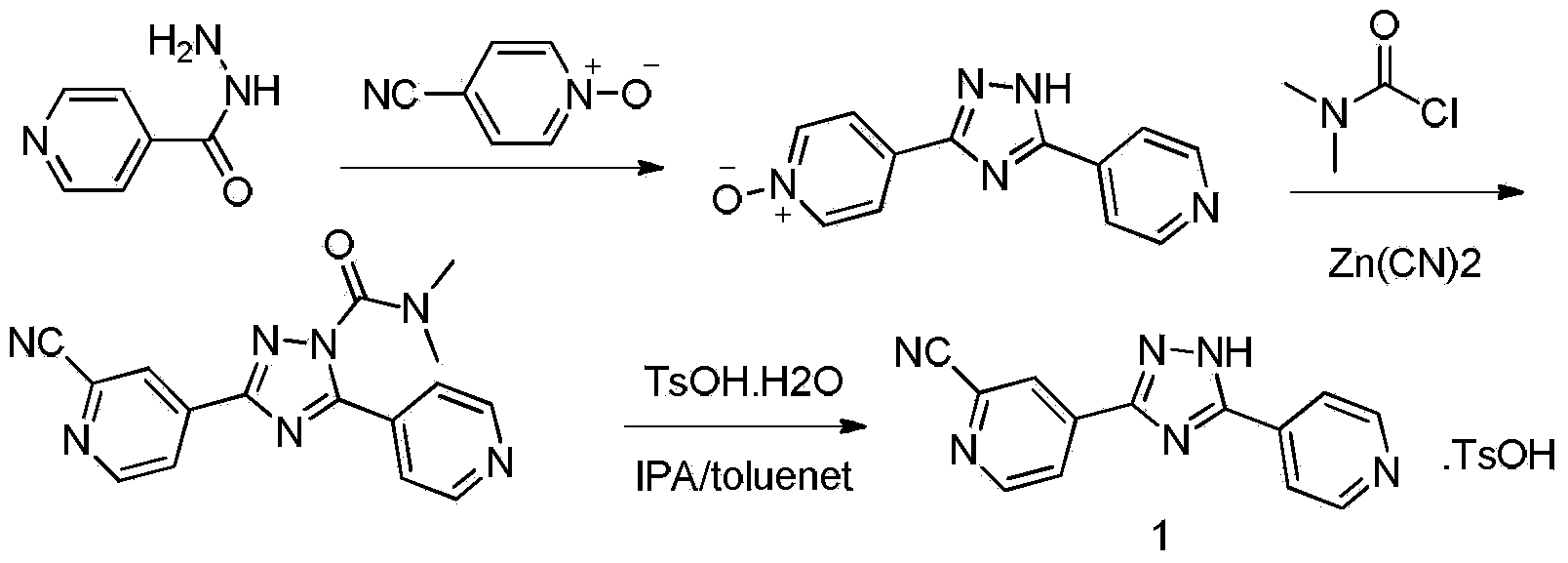
Preparation method of topiroxostat
A technology of topicastat and compounds, applied in the direction of organic chemistry and the like, can solve problems such as serious environmental pollution and large dosage, and achieve the effects of high product purity, reduced dosage, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the preparation of formula (4) compound 4-(5-(pyridin-4-yl)-1H-1,2,4-triazol-3-yl)pyridine-N-oxide
[0037] Dissolve 1.20 g (0.01 mol) of 4-cyanopyridine-N-oxide (compound of formula 5) in 50 ml of methanol, then add 50 mg of sodium methoxide, and stir until dissolved. Then 1.37 g (0.01 mol) of isoniazid (compound of formula 6) was added, and heated to reflux for 10 hours. After the reaction, the precipitated solid was filtered out, washed with methanol, and then dried with a vacuum pump to obtain 1.87 g of a yellow powder product, the compound of formula (4), with a yield of 78%.
Embodiment 2
[0038] Embodiment 2: the preparation of formula (4) compound 4-(5-(pyridin-4-yl)-1H-1,2,4-triazol-3-yl)pyridine-N-oxide
[0039] Dissolve 12.0 g (0.10 mol) of 4-cyanopyridine-N-oxide (compound of formula 5) in 500 ml of methanol, then add 1.0 g of sodium methoxide, and stir until dissolved. Then, 13.7 g (0.10 mol) of isoniazid (compound of formula 6) was added, and heated to reflux for 15 hours. After the reaction, the precipitated solid was filtered out, washed with methanol and dried with a vacuum pump to obtain 18.2 g of the compound of formula (4) as a yellow powder product with a yield of 77%.
Embodiment 3
[0040] Embodiment 3: Formula (3) compound 3-(2-cyanopyridin-4-yl)-N, N-dimethyl-5-(pyridin-4-yl)-1H-1,2,4-three Preparation of azole-1-carboxamides.
[0041]With formula (4) compound 2.39g (10mmol), dimethylcarbamoyl chloride (0.22ml, 22mmol), zinc cyanide (1.40g, 12mmol), cuprous iodide 0.19g (1mmol) is dissolved in 100ml acetonitrile, the mixture React at 80°C for 5 hours, TLC detects the reaction (n-hexane / ethyl acetate=1:1), after the reaction is complete, cool to room temperature, add 100ml of water and stir for 5-30 minutes, discard the organic layer, and use the water layer Ethyl acetate was extracted three times, 100ml each time, the organic layers were combined, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain the crude product, which was subjected to column chromatography with n-hexane / ethyl acetate=4:1 to obtain a pale yellow solid 2.97 g, yield 93%, HPLC purity (normalization method): 97.7%; melting point: 112...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com