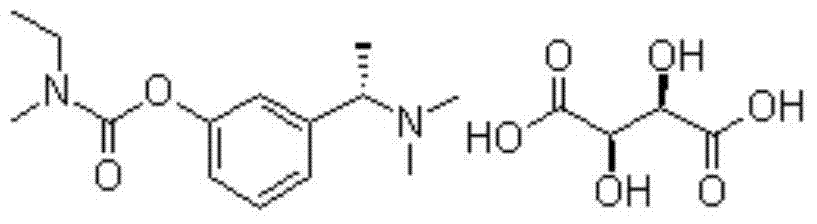
Production process of rivastigmine
A synthesis process and a dimethylamino technology are applied in the field of synthesis technology of rivastigmine, can solve the problems of low yield, long reaction steps, complicated reaction operations, etc., and achieve the advantages of simplifying production process, improving practicability and reducing cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0018] The synthesis of embodiment 13-(1-(dimethylamino) ethyl) phenol
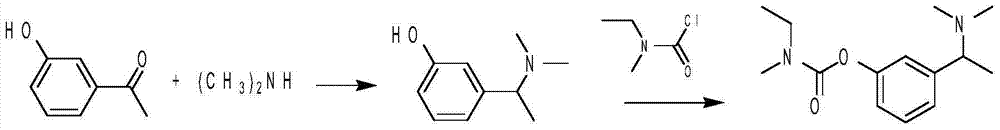
[0019] Under the condition of ice-water bath, slowly add 54.5g (0.4mol) of 33% dimethylamine aqueous solution into a 250ml three-necked flask containing 20.9g (0.4mol) of 88w% formic acid. 46ml to obtain dimethylammonium formate. When the temperature drops below 100°C, add m-hydroxyacetophenone 13.6g (0.1mol), formic acid (concentration 88w%) 4.6g (0.1mol), MgCl 2 ·6H 2 O3g (0.015mol). Raise the temperature to 170°C, heat and react for 5 hours. After the reaction is completed, pour the reaction solution into 80ml of water, wash the reaction bottle with about 5ml of water and combine them together, adjust the pH value to 1-2 with concentrated hydrochloric acid, filter, extract with ether, and use ether for phase use Drying over anhydrous magnesium sulfate and rotary evaporating to dryness yielded 6.47 g of m-hydroxyacetophenone as a solid raw material for recycling. The aqueous phase was adjusted to pH...
Embodiment 2
[0020] The synthesis of embodiment 2 rivastigmine
[0021] Add 1.65g (0.01mol) of 3-(1-(dimethylamino)ethyl)phenol, 0.42g (0.0105mol) of 60% NaH, and 15ml of tetrahydrofuran into a 50ml flask. After mixing thoroughly, add methylethylcarbamoyl chloride 1.28g. Stir at room temperature for 2 hours, recover THF, add diethyl ether for extraction, wash with 0.1mol / L NaOH solution, wash with water, evaporate diethyl ether, and dry under reduced pressure at 40°C to constant weight to obtain a yellow liquid rivastigmine free base (2.34g, 93.6%).
[0022]
Embodiment 3
[0023] The resolution of the rivastigmine of embodiment 3 racemic
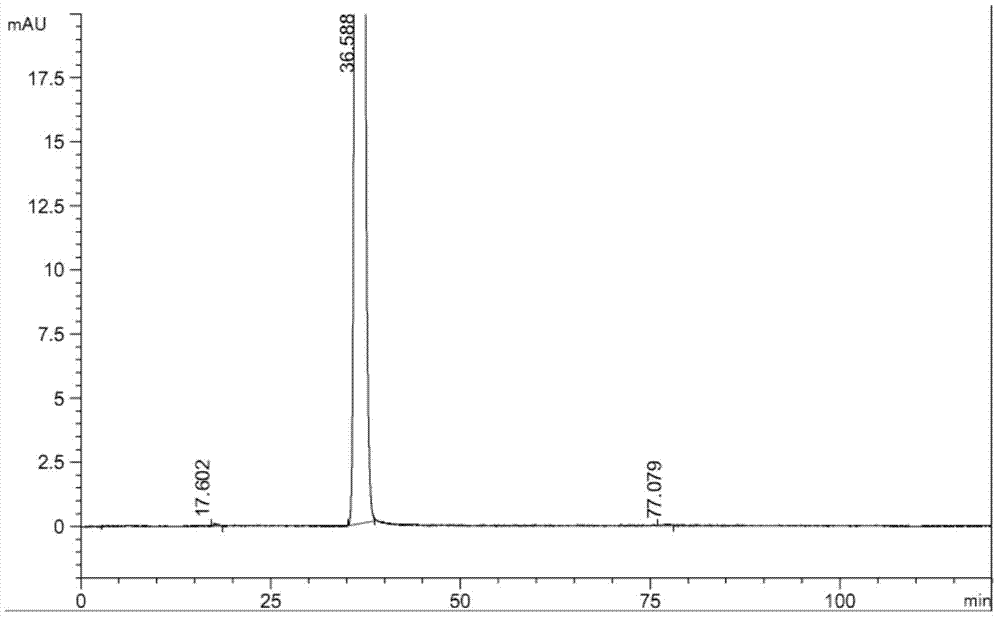
[0024] Add 40ml of methanol / water (2:1) and 4g of racemic rivastigmine and 6.2g of D-(+)-p-methyldibenzoyl tartaric acid into a 100ml flask, heat to reflux to dissolve, and cool White crystals precipitated out. Repeat crystallization three times to obtain 3.3 g of DTTA salt of rivastigmine
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com