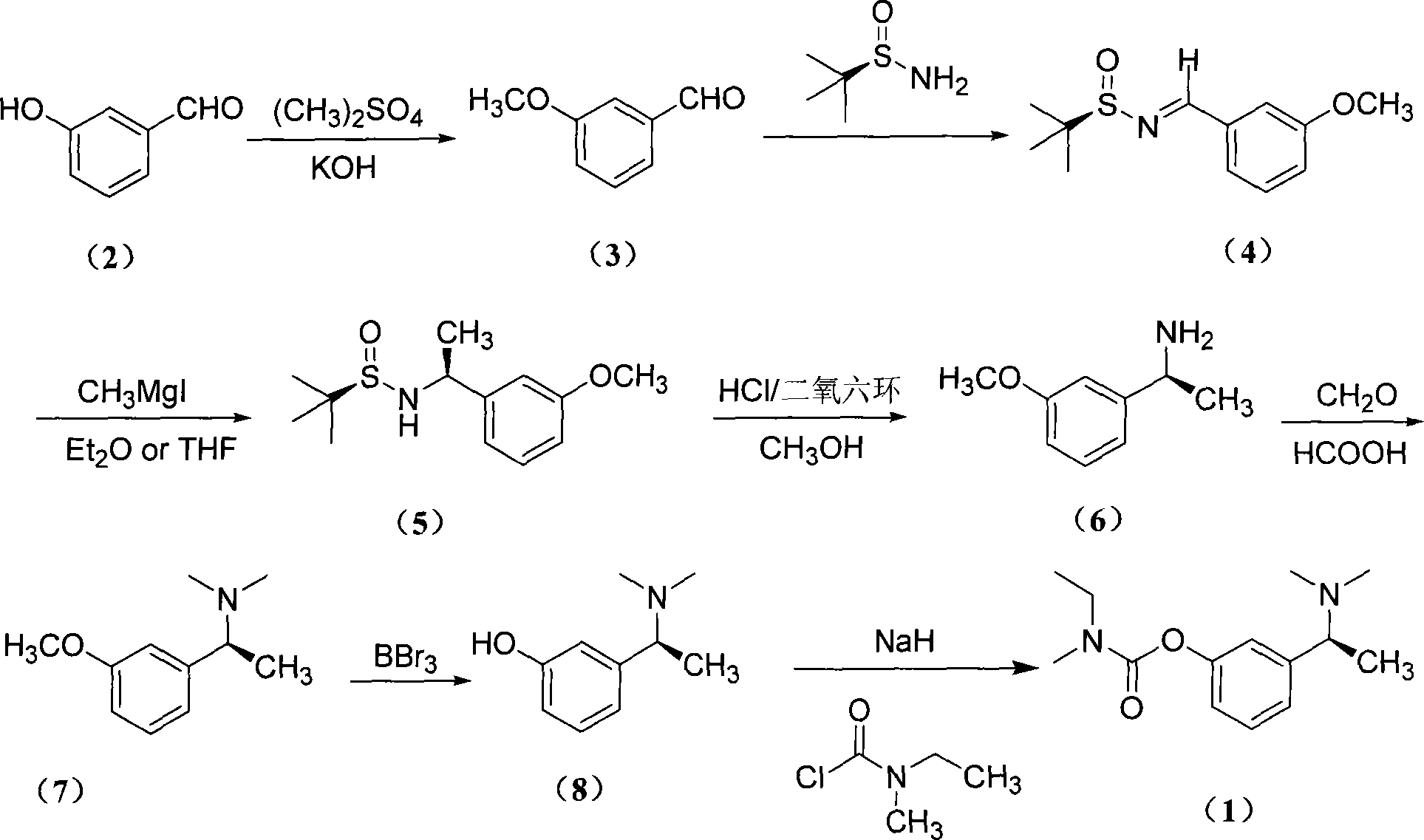
Asymmetric synthesis method of (S)-rivastigmine
A synthetic method and asymmetric technology, applied in the field of chiral drug synthesis, can solve the problems of no rivastigmine and other problems, and achieve the effects of easy availability of raw materials, cost reduction, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Synthesis of 3-methoxybenzaldehyde (3)
[0040] In a 250ml three-necked flask, add 3-hydroxybenzaldehyde (6.10g, 50mmol) and 15ml of methanol solution, heat to about 65°C under magnetic stirring, and dropwise add dimethyl sulfate (7.41ml, 75mmol) and potassium hydroxide Solution (KOH (5.04 g, 90 mmol), 8 ml water). Continue to stir the reaction for 24h after the addition, stop the reaction, and cool to room temperature. Extracted with ethyl acetate (15ml×3), dried over anhydrous sodium sulfate, rotary evaporated to dryness, and column chromatography gave 3-methoxybenzaldehyde (3) (5.76g, yield: 84.1%) as a yellow oil.
[0041] (2) Synthesis of (R, E)-3-methoxy-benzylidene tert-butylsulfonamide (4)
[0042] Under nitrogen protection, weigh tert-butylsulfinamide (2.40g, 20mmol) and anhydrous copper sulfate (7.00g, 44mmol), put tert-butylsulfinamide into a 100ml three-necked bottle, add 30ml dichloro Methane, anhydrous copper sulfate was added under magnetic stirrin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com