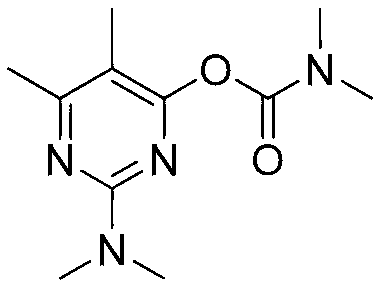
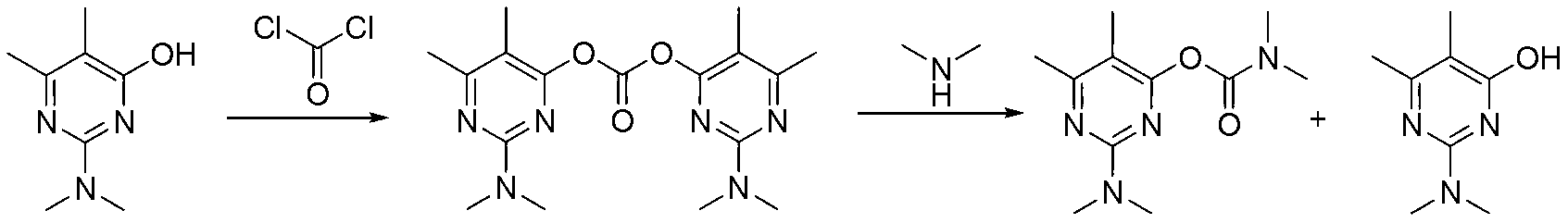
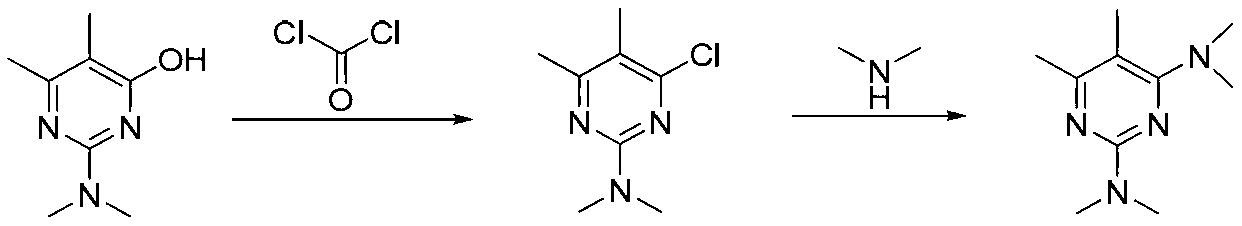
Preparation method of pirimicarb
A pirimicarb-resistant and dimethyl-resistant technology is applied to the preparation field of pirimicarb-resistant, can solve the problems of low yield, long reaction time and the like, and achieves the effects of short reaction time, low production cost, simple operation and post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 34.1g (0.2mol) of 5,6-dimethyl-2-dimethylamino-4-hydroxypyrimidine (98%) into a 500mL three-necked flask with cooling tube, water separator, dropping funnel, thermometer, and stirring. 340g of toluene and 8.4g (0.2mol) of sodium hydroxide were stirred and heated to the reflux temperature, and the water generated by the reaction was removed from the system. After no obvious water was observed, the reflux was continued for 0.5hr (moisture content was less than 0.1% for sampling analysis). Cool down to 50°C, remove the water separator, add 0.3g of 4-dimethylaminopyridine and 24.1g (0.22mol) of dimethylcarbamoyl chloride into the kettle, stir and raise the temperature to 70°C for 3 hours, stop the reaction and cool down. Add 100mL of water to wash and separate the phases, and the organic phase is precipitated under negative pressure to obtain 47.8g. The content of pirimicarb is 98.6% according to the external standard method of liquid chromatography, and the yield is 99....
Embodiment 2
[0030] Add 34.1g (0.2mol) of 5,6-dimethyl-2-dimethylamino-4-hydroxypyrimidine (98%) into a 500mL three-necked flask with cooling tube, water separator, dropping funnel, thermometer, and stirring. 204.6g of toluene and 8.4g (0.2mol) of sodium hydroxide, stirred and heated to reflux temperature, removed the water generated by the reaction from the system, and continued to reflux for 0.5hr after no obvious water was produced (moisture content was less than 0.1% for sampling analysis) . Cool down to 50°C, remove the water separator, add 0.6g of 4-dimethylaminopyridine and 25.2g (0.23mol) of dimethylcarbamoyl chloride into the kettle, stir and raise the temperature to 70°C for 3 hours, stop the reaction and cool down. Add 100mL of water to wash and separate the phases, and the organic phase is precipitated under negative pressure to obtain 47.8g. The content of pirimicarb is 98.3% according to the external standard method of liquid chromatography, and the yield is 98.7%.
Embodiment 3
[0032] Add 34.1g (0.2mol) of 5,6-dimethyl-2-dimethylamino-4-hydroxypyrimidine (98%) into a 500mL three-necked flask with cooling tube, water separator, dropping funnel, thermometer, and stirring. 272.8g of toluene and 8.4g (0.2mol) of sodium hydroxide, stirred and heated to reflux temperature, removed the water generated by the reaction from the system, and continued to reflux for 0.5hr after observing that no obvious water was produced (moisture content was less than 0.1% in sampling analysis) . Cool down to 50°C, remove the water separator, add 0.9g of 4-dimethylaminopyridine and 28.5g (0.26mol) of dimethylcarbamoyl chloride into the kettle, stir and raise the temperature to 70°C for 2 hours, stop the reaction and cool down. Add 100mL of water to wash and separate the phases, and the organic phase is precipitated under negative pressure to obtain 47.9g. The content of pirimicarb is 98.8% according to the external standard method of liquid chromatography, and the yield is 99....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com