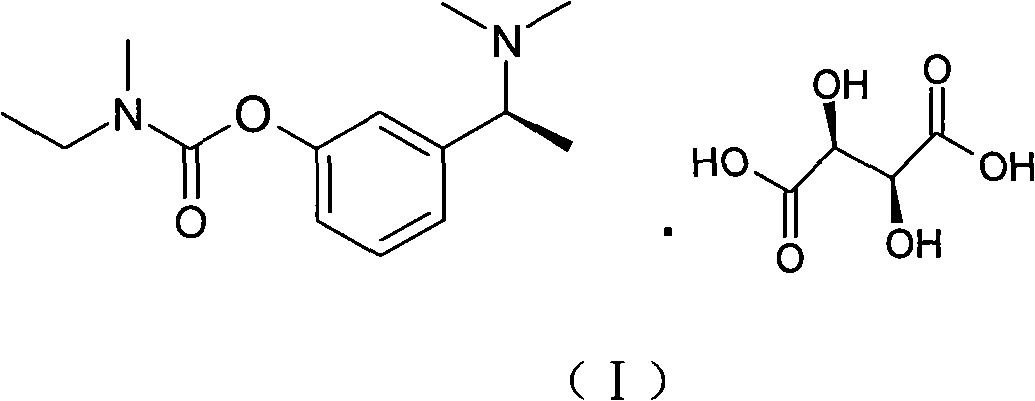
Synthetic method of rivastigmine and intermediates thereof
A technology of rivastigmine tartrate and compounds, which is applied in the field of anti-senile dementia drugs, can solve the problems of complex operation, low yield, and many by-products, and achieve the effects of simple and safe operation, simple process operation, and few reaction by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
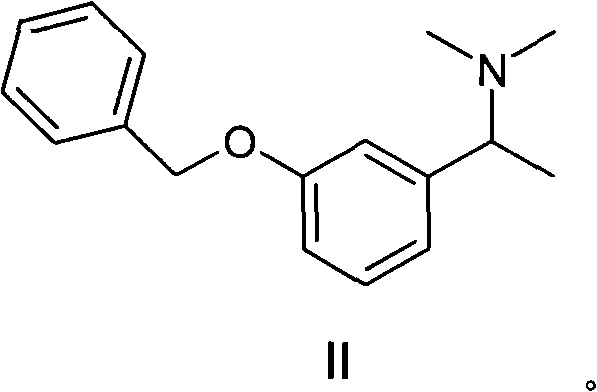
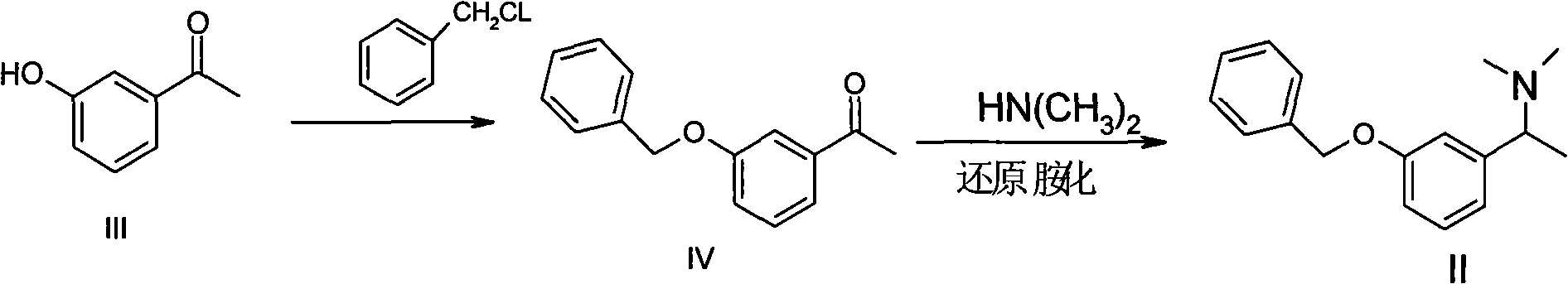
[0032] Example 1 Preparation of [1-(3-benzyloxy-phenyl)-ethyl]-dimethylamine (II)
[0033] (1) Preparation of 3-benzyloxyacetophenone (IV)
[0034] Add 3-hydroxyacetophenone (20g, 0.15mol) and 80ml of dimethylformamide into a 250ml reaction bottle, start stirring, after dissolving, add potassium carbonate (0.22mol), heat the oil bath to about 70°C, start Benzyl chloride (19.4g, 0.15mol) was added dropwise, and the temperature was controlled at 70-80°C during the dropwise addition. Acetone:cyclohexane=1:1 (v / v), ultraviolet color development), start to subtract the solvent, add 100ml water and 50ml ethyl acetate to the residual liquid, stir fully to separate the organic layer, and then use 50ml acetic acid to separate the water layer Extract once with ethyl ester, combine the organic layers, wash with 50ml of water × 3 times, add anhydrous magnesium sulfate to dry, evaporate the solvent to obtain the target product 3-benzyloxyacetophenone (30g), the yield is 93%. 1 H-NMR, MS ...
Embodiment 2
[0041] Example 2 Preparation of [1-(3-benzyloxy-phenyl)-ethyl]-dimethylamine (II)
[0042] (1) Preparation of 3-benzyloxyacetophenone (IV)
[0043] Add 3-hydroxyacetophenone (20g, 0.15mol) and 70ml of dichloroethane into a 250ml reaction bottle, start stirring, after dissolving, add potassium carbonate (0.22mol), heat the oil bath to about 50°C, and start dripping Add benzyl chloride (19.4g, 0.15mol), and control the temperature at 50-60°C during the dropwise addition process. After the dropwise addition, continue to keep warm at about 60°C for about 24 hours. : Cyclohexane=1:1 (v / v), ultraviolet color development), began to subtract the solvent, added 100ml water and 50ml ethyl acetate in the residual liquid, stirred fully to separate the organic layer, and then washed the water layer with 50ml ethyl acetate The ester was extracted once, the organic layers were combined, washed with 50ml of water × 3 times, dried by adding anhydrous magnesium sulfate, and the solvent was eva...
Embodiment 3
[0050] Example 3 Preparation of [1-(3-benzyloxy-phenyl)-ethyl]-dimethylamine (II)
[0051] (1) Preparation of 3-benzyloxyacetophenone (IV)
[0052] Add 3-hydroxyacetophenone (20g, 0.15mol) and 80ml toluene into a 250ml reaction bottle, start stirring, after dissolving, add sodium carbonate (36.3, 0.22mol), heat the oil bath to about 80°C, and start adding dropwise Benzyl chloride (19.4g, 0.15mol), the temperature is controlled at 80-90°C during the dropwise addition, and after the dropwise addition, continue to insulate and react at about 90°C for about 20 hours. Cyclohexane=1:1 (v / v), ultraviolet color development), start to subtract the solvent, add 100ml water and 50ml ethyl acetate to the residual liquid, stir fully to separate the organic layer, and then use 50ml ethyl acetate Extract once, combine the organic layers, wash with 50ml of water × 3 times, add anhydrous magnesium sulfate to dry, evaporate the solvent to obtain the target product 3-benzyloxyacetophenone (32.3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com