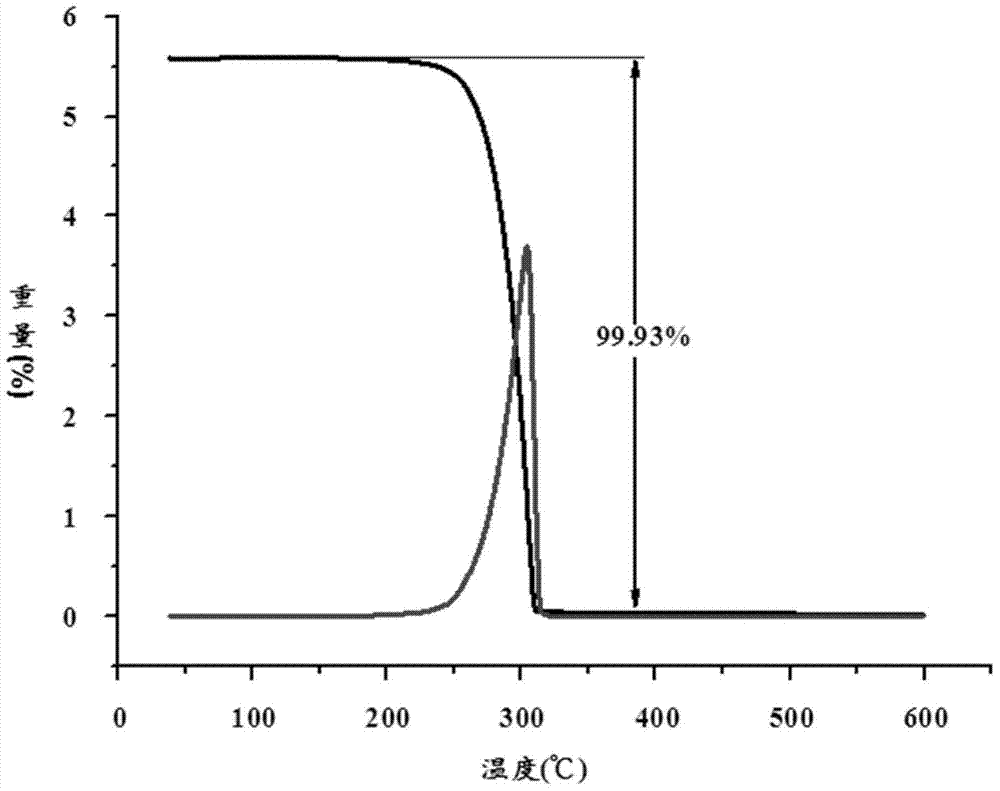
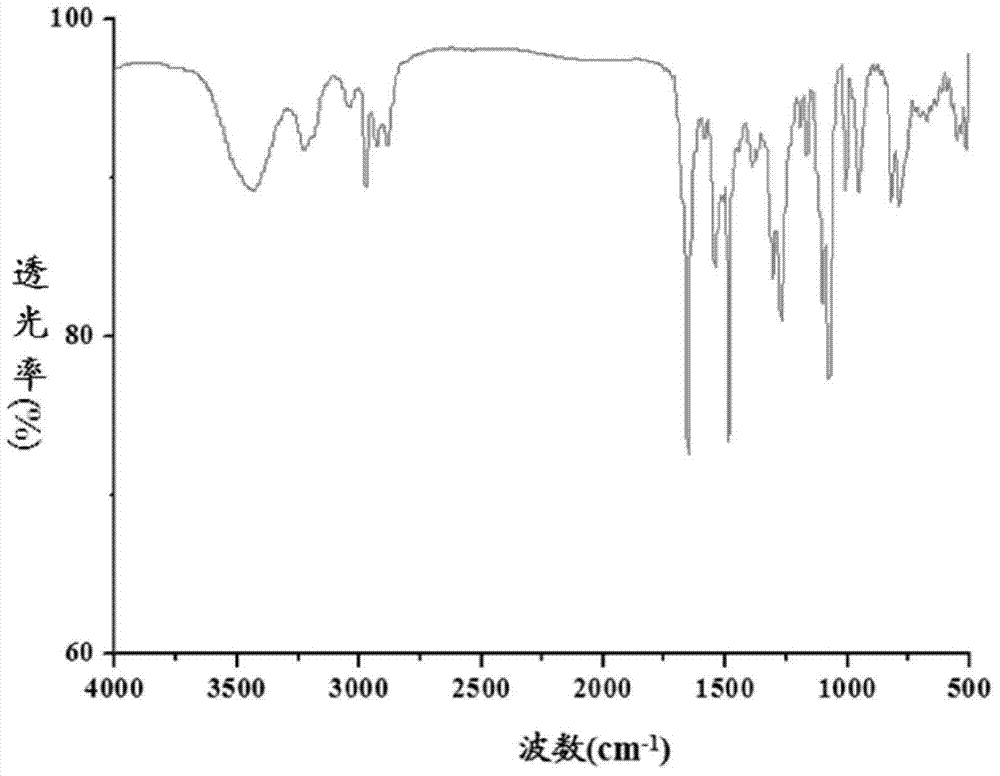
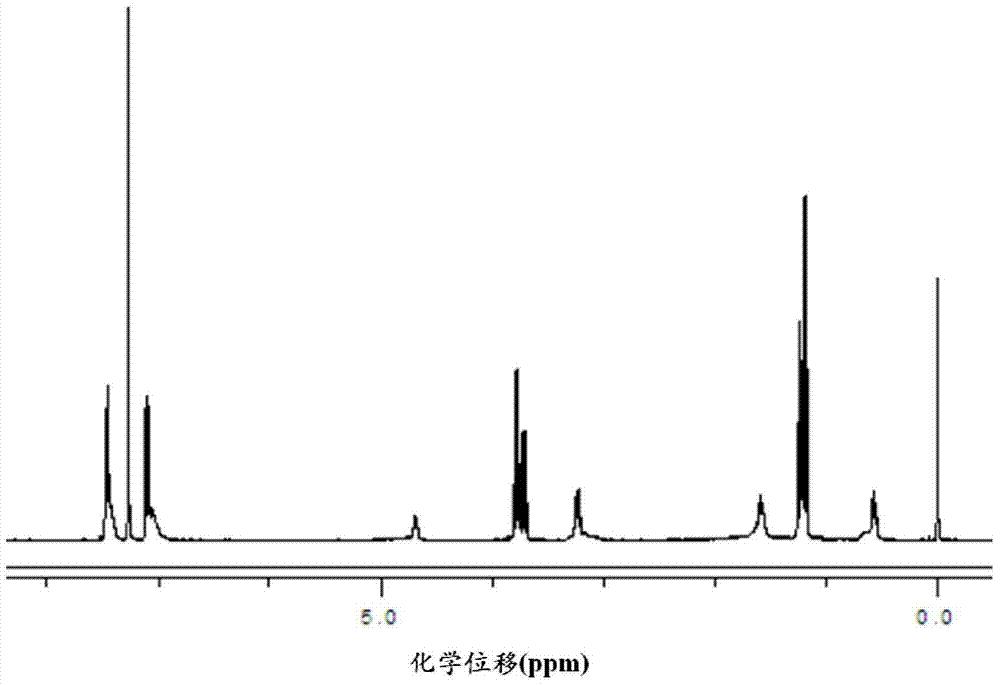
Functional organosiloxane containing asymmetrical substituted urea and preparation method thereof
A technology of organosiloxane and substituted urea, which is applied in the field of functional organosiloxane and its preparation, can solve the problems of single special function and rare varieties, and achieves a product with good interfacial compatibility, easy hydrolysis and high conversion rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesize 1,1-bis(4-bromobenzene)-3-(3-triethoxysilyl)propylurea according to the following method, and the synthetic route is as follows:
[0034]
[0035] Concrete synthetic steps are as follows:
[0036] (1) N,N -Synthesis of two (4-bromophenyl) carbamoyl chlorides
[0037] In a 100ml three-neck flask equipped with electromagnetic stirring and temperature control device, 4,4 , -Dibromodiphenylamine (0.8175g, 2.5mmol) was dissolved in 50ml of dichloromethane, continued to add triethylamine (1.515g, 15mmol), then added solid triphosgene (2.673g, 9mmol), refluxed and stirred at room temperature, The reaction was detected by TLC tracking, and the reaction was stopped after 24 hours. After the reaction was over, dichloromethane was distilled off under reduced pressure, and the crude product was separated and purified by column chromatography (petroleum ether: dichloromethane=2:1) to obtain 0.84 g of a light yellow solid intermediate, which was purified by 1...
Embodiment 2
[0046] Synthesis of 1,1-bis(4-bromophenyl)-3-(3-trimethoxysilyl)propylurea
[0047] In a 100ml three-necked flask equipped with electromagnetic stirring, the intermediate N,N-bis(4-bromophenyl)carbamoyl chloride (0.934g, 2.4mmol), 50ml of ether, and triethylamine (0.606g , 6mmol), 3-aminopropyltrimethoxysilane (0.72g, 4mmol), pass into N 2 Protection, TLC detection reaction, stop after 12 hours at room temperature. After the reaction, the white precipitate was filtered off, the filtrate was distilled off under reduced pressure to remove ether, and the crude product was separated and purified by column chromatography (petroleum ether: ethyl acetate = 3:1) to obtain 1.08 g of a light yellow solid product with a yield of 84.4%. The infrared spectrum, ultraviolet spectrum of example 1, 1 H-NMR and 13 C-NMR determined it to be 1,1-bis(4-bromophenyl)-3-(3-trimethoxysilyl)propylurea.
Embodiment 3
[0049] Synthesis of 1,1-diphenyl-3-(3-triethoxysilyl)propylurea
[0050] In a 100ml three-necked flask equipped with electromagnetic stirring and a temperature control device, diphenylamine (1.69g, 10mmol) was dissolved in 50ml of dichloromethane, triethylamine (3.03g, 30mmol) was added continuously, and then solid triphosgene (5.346 g, 18mmol), reflux and stir at room temperature, TLC detection reaction, stop the reaction after 24h. After the end of the reaction, dichloromethane was distilled off under reduced pressure to obtain the intermediate N,N -diphenylcarbamoyl chloride; add the N,N -diphenylcarbamoyl chloride (1.15g, 5mmol), ether 50mL, pyridine (0.48g, 6mmol), 3-aminopropyl triethoxysilane (1.1g, 5mmol), pass into N 2 Protection, TLC detection reaction, stop after 12 hours at room temperature. After the reaction, the yellow precipitate was filtered off, the filtrate was distilled off under reduced pressure to remove diethyl ether, and the crude product was separ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com