Chiral intermediate of rivastigmine, and preparation method thereof
A chiral reagent, ethyl technology, applied in the field of rivastigmine chiral intermediate and preparation thereof, can solve problems such as unfavorable industrial production, immature method, low total yield, etc. The effect of reducing the burden of three waste treatment and reducing the loss of splitting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046]
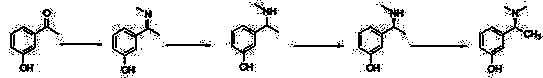
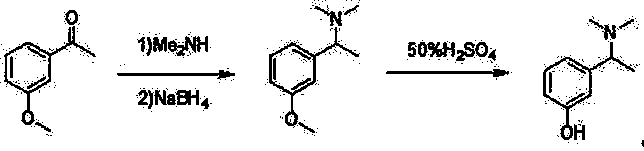
[0047]1) In a 500 mL three-neck flask equipped with a thermometer, add 50 g of 3-hydroxyacetophenone and 100 mL of methanol into the flask, add 44.7 g of methylamine aqueous solution (28%) dropwise, stir for 3 h, and filter to obtain a yellow Solid 3-(1-methylimino)ethyl)phenol 41.1 g, yield 75%;
[0048]
[0049] 2) Add 45 g of the above-mentioned 3-(1-methylimino)ethyl)phenol into 500 mL of absolute ethanol, add 8.1 g of potassium borohydride solid at room temperature, and stir at 30°C for 2 h, HPLC detects that the reaction of 3-(1-methylimino)ethyl)phenol is complete, add 500 mL of water, filter out inorganic salts, concentrate absolute ethanol, add about 500 mL of water, stir for 3 h, and filter to obtain white Solid, dried to obtain 3-(1-methylamino)ethyl)phenol 34.2 g, yield 75%;
[0050]
[0051] 3) Add 30 g of 3-(1-methylamino)ethyl)phenol and 46 g of D-camphorsulfonic acid into 250 mL of tetrahydrofuran (in, heat to reflux, completely dissolve, slo...
Embodiment 2
[0055] 1) In a 500 mL three-neck flask equipped with a thermometer, add 50 g of 3-hydroxyacetophenone and 50 mL of isopropanol into the flask, add 48.8 g of methylamine aqueous solution (28%) dropwise, stir for 6 h, and filter Obtained 46.6 g of yellow solid 3-(1-methylimino)ethyl)phenol, with a yield of 85%;
[0056] 2) Add 45 g of the above-mentioned 3-(1-methylimino)ethyl)phenol into 600 mL of anhydrous methanol, control the temperature at 40 °C, add 11.4 g of sodium borohydride solid, and Stir at low temperature for 2 h, HPLC detects that the reaction of 3-(1-methylimino)ethyl)phenol is complete, add 300 mL of water, filter off inorganic salts, concentrate anhydrous methanol, add about 500 mL of water, and stir for 8 h. A white solid was obtained by filtration and dried to obtain 31.9 g of 3-(1-methylamino)ethyl)phenol, with a yield of 70%;
[0057] 3) Add 30 g of 3-(1-methylamino)ethyl)phenol and 29.7 g of d-tartaric acid into 150 mL of isopropanol, heat to reflux, disso...
Embodiment 3
[0060] 1) In a 500 mL three-neck flask equipped with a thermometer, add 50 g of 3-hydroxyacetophenone and 200 mL of ethanol into the flask, add 52.8 g of methylamine aqueous solution (28%) dropwise, stir for 9 h, and filter to obtain a yellow Solid 3-(1-methylimino)ethyl)phenol 43.9 g, the yield is 80%;
[0061] 2) Add 45 g of the above-mentioned 3-(1-methylimino)ethyl)phenol into 600 mL of anhydrous isooctyl alcohol, control the temperature at 50 °C, and add 15.2 g of solid sodium cyanoborohydride , stirred at 50°C for 6 h, HPLC detected that the reaction of 3-(1-methylimino)ethyl)phenol was complete, added 300 mL of water, filtered off inorganic salts, concentrated anhydrous isooctyl alcohol, and added about 500 mL water, stirred for 4 h, filtered to obtain a white solid, and dried to obtain 36.5 g of 3-(1-methylamino)ethyl)phenol, with a yield of 80%;
[0062] 3) Add 30 g of 3-(1-methylamino)ethyl)phenol and 30.2 g of D-mandelic acid into 300 mL of ethanol, heat to reflux,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com