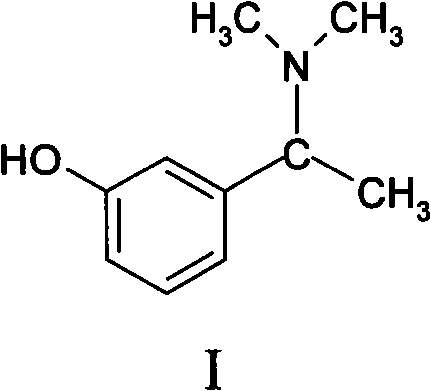
Preparation method for Rivastigmine midbody 3-hydroxyl-alpha-N, N-dimethyl phenylethylamine
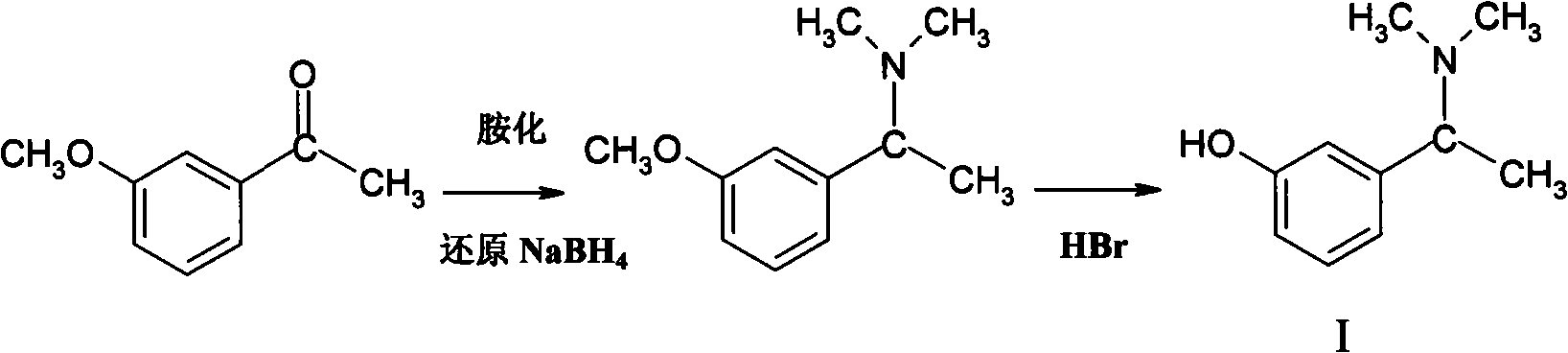
A technology of dimethylphenethylamine and hydroxyacetophenone, which is applied in the field of drug synthesis, can solve problems such as long reaction time, consumption, and influence on product yield and quality, and achieve simple methods, high product purity, and low reaction pollution little effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
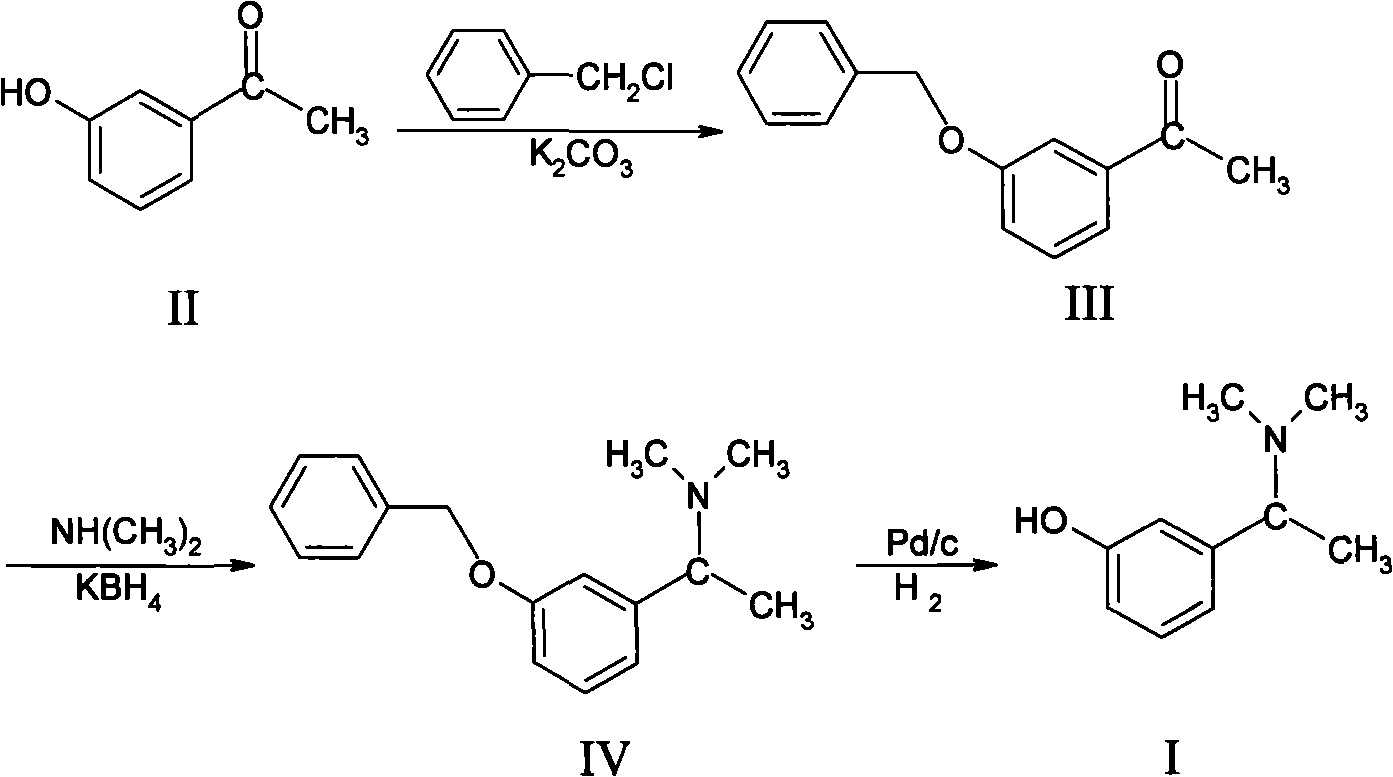
[0016] Example 1 Preparation of 3-benzyloxyacetophenone (III)
[0017] In a 500ml dry reaction flask, put 50g (0.37mol) of m-hydroxyacetophenone, 300g of DMF and 101.4g (0.73mol) of potassium carbonate, stir, add 60g (0.47mol) of benzyl chloride dropwise, raise the temperature to 70°C, and keep it warm for 2 hours , DMF was recovered under reduced pressure, an appropriate amount of water was added, and extracted with toluene, the aqueous layer and the organic layer were recovered to exhaust the solvent, and 82.5 g of the product was obtained. Yield: 99.28%,. h 1 NMR (δ2.5, s 3H; δ5.05, s 2H; δ7.1-7.69H)
Embodiment 2
[0018] Example 2 Preparation of 3-benzyloxy-α-N, N-dimethylaminophenethylamine (IV)
[0019] In a 1000ml dry reaction bottle, put 250g of absolute ethanol into it, cool it to about 0°C with an ice bath, pass through 60g of dimethylamine, and 2 Under protective condition, drip tetraisopropoxytitanium 190g (0.67mol), then add m-benzyloxyacetophenone 83g (0.37mol) that embodiment 1 makes, keep warm at room temperature for 10 hours, slowly add potassium borohydride, keep warm After 12 hours, add ammonia water, filter, and concentrate the filtrate under reduced pressure, add hydrochloric acid to the residue to make it acidic, extract with toluene, and adjust the pH of the water layer with NaOH solution to 12-14, extract with toluene again, and separate the water layer , and the toluene layer was concentrated under reduced pressure to obtain 80 g of light yellow oil. Yield: 85.42%, H 1 NMR (δ1.3, d3H; δ2.08, s 6H; δ3.1 1H; δ5.0 2H; δ6.8-7.5 9H)
Embodiment 3
[0020] Example 3 3-Hydroxy-α-N, the preparation of N-dimethylphenethylamine (I)
[0021] In a 1000ml dry reaction flask, drop into 700g ethanol, 80g of the product obtained in Example 2, stir and add dropwise 38% 40g hydrochloric acid and 5g palladium-carbon, under normal pressure, feed hydrogen for 6 hours, temperature 35~ 40°C, stop hydrogen flow, filter, concentrate the ethanol to the filtrate under reduced pressure, add 100ml of water, adjust the pH to 9.5 with 10% NaOH, add toluene, separate layers, combine the toluene layers, concentrate the toluene under reduced pressure, and cool the residue to crystallize. Filter and dry to obtain 42 g of light yellow solid. Yield: 81.1%, H 1 NMR (δ 1.4, d 3H; δ 2.21, s 6H; δ 3.3 1H; δ 6.7-7.2 3H). The content detected by HPLC was 99.20%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com