Cholinesterase Inhibitors In Liposomes And Their Production And Use
a technology of cholinesterase inhibitors and liposomes, which is applied in the direction of biocide, drug compositions, active ingredients of phosphorous compounds, etc., can solve the problems of insufficient local ngf concentration, less successful studies in this regard, and blockage or enhancement of the effect of acetylcholine on the receptor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
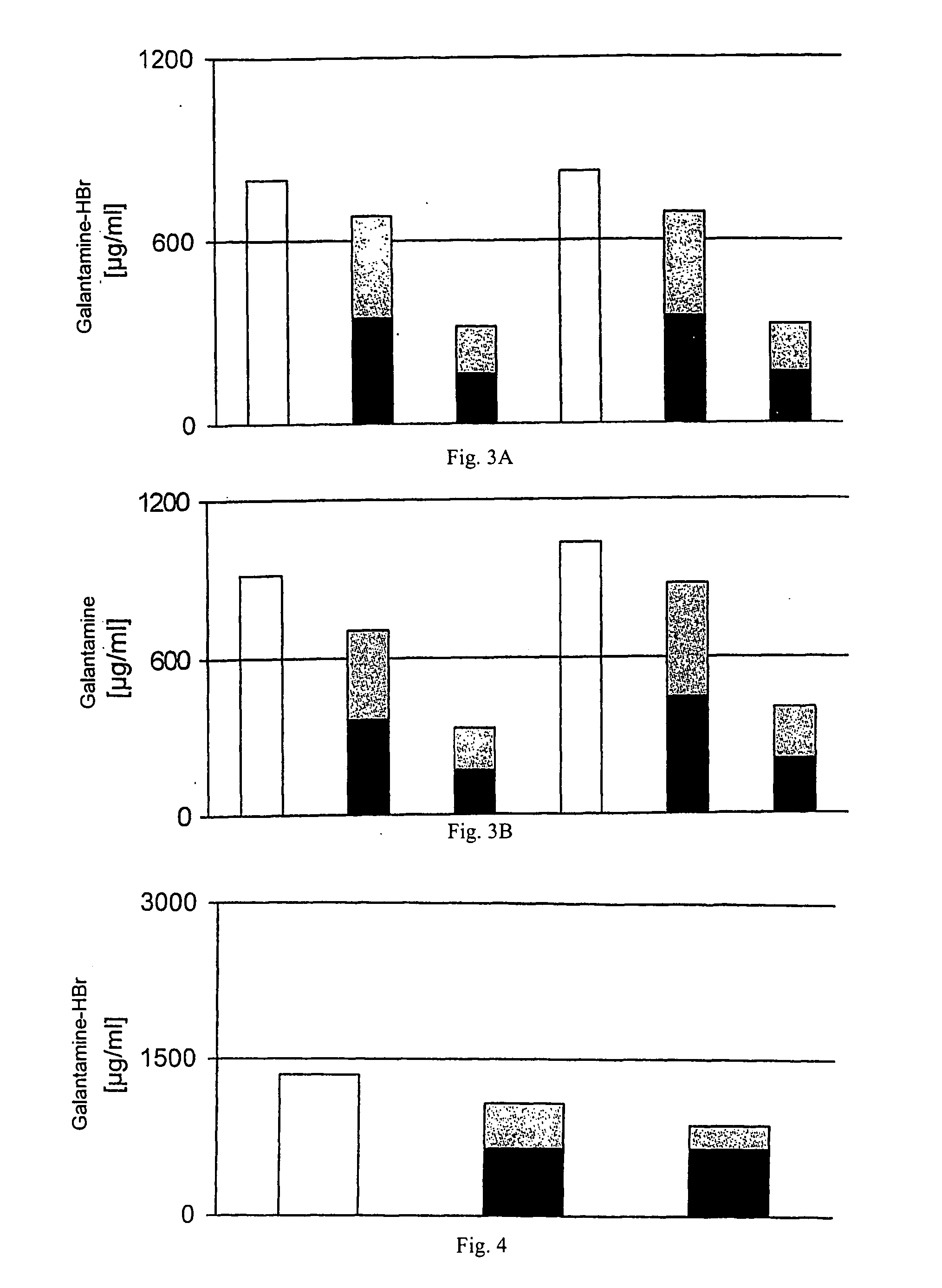
Preparation and Stability Testing of Galantamine Liposomes
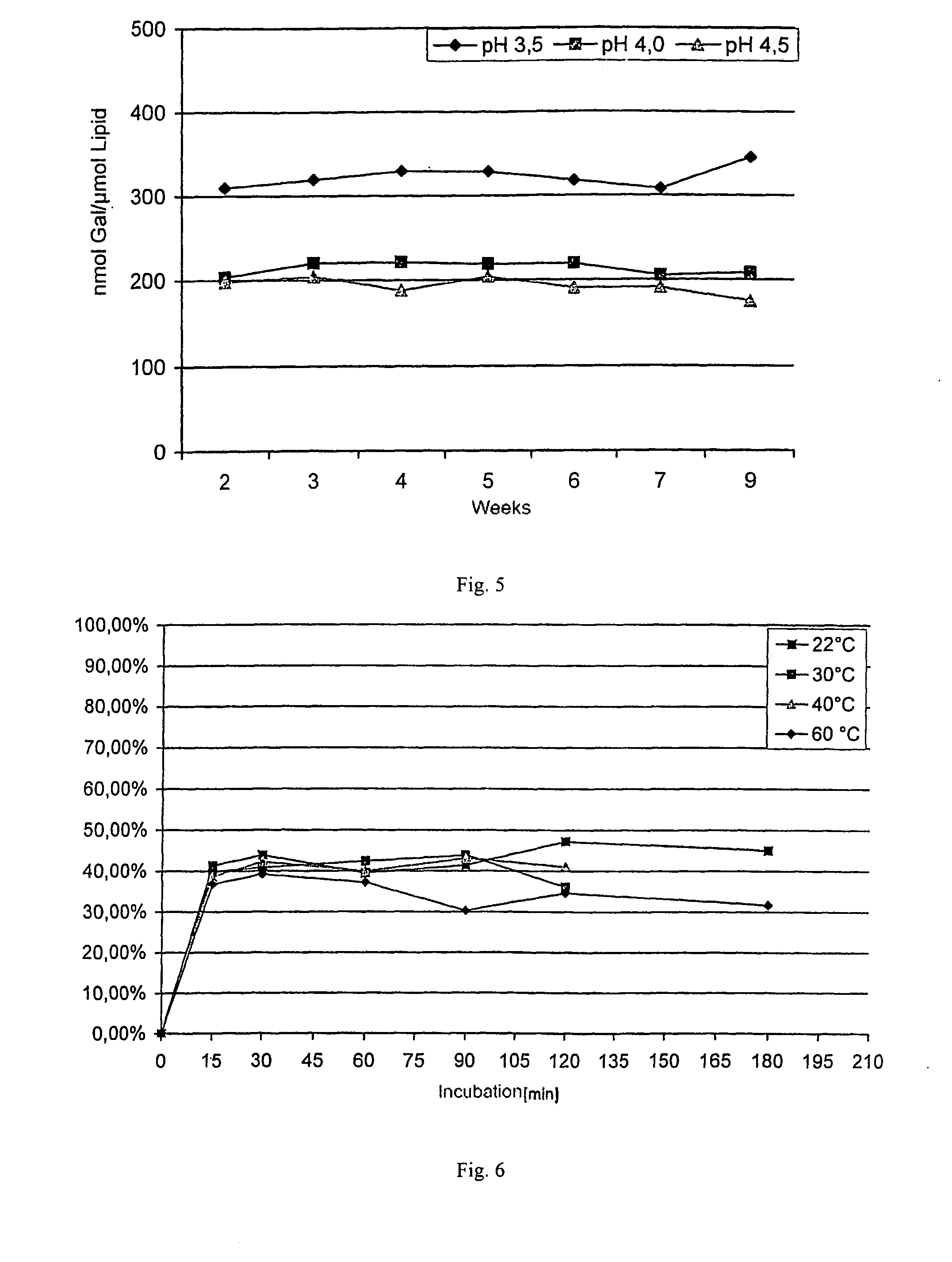
[0048] Synthetic dipalmitoylphosphatidylcholine (DPPC, Genzyme, Switzerland) and cholesterol (Solvay, Netherlands) were used to prepare vesicles. Some experiments were carried out either with hen's egg phosphatidylglycerol (E-PG; Lipoid Co., Germany) or with stearylamine (Sigma, USA), in order to introduce positive or negative charges into the liposomal membrane. Galantamine (Sanochemia AG, Austria) was used as the free base or as HBr salt in the inclusion experiments. PBS (phosphate buffered saline) or citric acid in combination with sodium carbonate were used as buffer solutions.
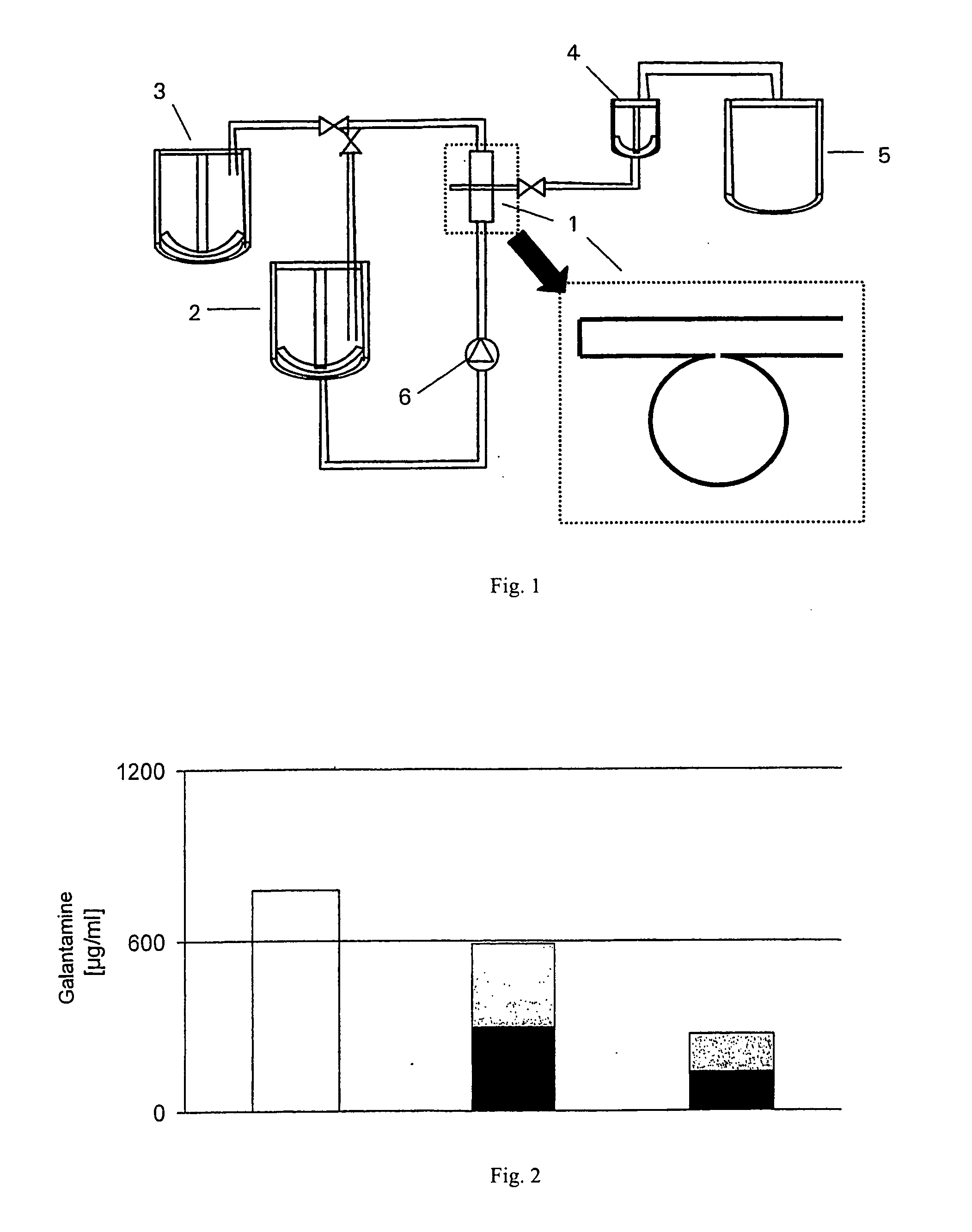
[0049] The liposomes were preferably prepared by means of the shear-free crossflow injection technique in accordance with WO 02 / 36257. This technique is highly reproducible and enables the inclusion of any active agents into liposomes. This continuous, one-step method allows unilamellar liposomes with a lipid double layer membrane (“bilayer”) with...
example 2
Preparation and Comparison of Galantamine Preparations in the Form of Liposomes or Microemulsions with Variable Lipid Composition
[0076] Synthetic dipalmitoylphosphatidylcholine (DPPC, Genzyme, Switzerland), dimyristoylphosphatidylcholine (DMPC, Genzyme, Switzerland) and cholesterol (Solvay, Netherlands) were used as lipids in this example. Galantamine (Sanochemia AG, Austria) was used as the HBr salt for the liposomal inclusion studies. Citric acid / sodium carbonate was used as buffer solution.
[0077] The ammonium sulfate gradient method was employed as a second possibility for active loading. An ammonium sulfate solution and a glucose solution were used as aqueous phases for vesicle preparation. Once again, the crossflow technique was used. After removing unenclosed galantamine by gel filtration, both the active agent and lipid contents were determined by rp-HPLC. The liposome size and size distribution were again determined by photon correlation spectroscopy (PCS).
[0078] In addit...
examples 3-10
Skin Penetration Tests of Various Lipid-Based Galantamine Preparations Franz Diffusion Cell
[0109] The diffusion cell came from PermeGear, USA. The equipment consisted of three diffusion cells, each with a water-filled double jacket mounted on an agitator bracket and connected to a water bath for temperature control. The cells themselves had a receptor volume of 8 mL each, a skin holder with a surface area of 0.78 cm2 and a donor chamber with 2 mL volume.
[0110] Skin Integrity Test:
[0111] Pigskin was used for the tests in the Franz diffusion cell. In order to ensure an intact skin surface, each skin piece was tested before and after the experiment. After affixing the skin on the skin holder of the diffusion cell, 2 mL buffer was applied to the skin and it was heated to 32±1° C. After 30 minutes, the electrical conductivity, a measure of the resistance of the skin and quality of the skin, was measured. The measurement value is dependent on the origin of the skin, its thickness, the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mol % | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| mol % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com