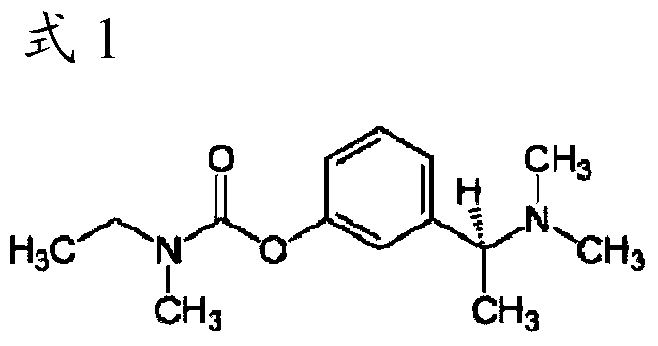
Percutaneous absorption preparation containing rivastigmine
A rivastigmine and preparation technology, applied in the field of percutaneous absorption preparations, can solve problems such as difficulty in obtaining effective efficacy and poor skin penetration, achieve reduced skin irritation, good adhesion and wear performance, and simplify the preparation process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
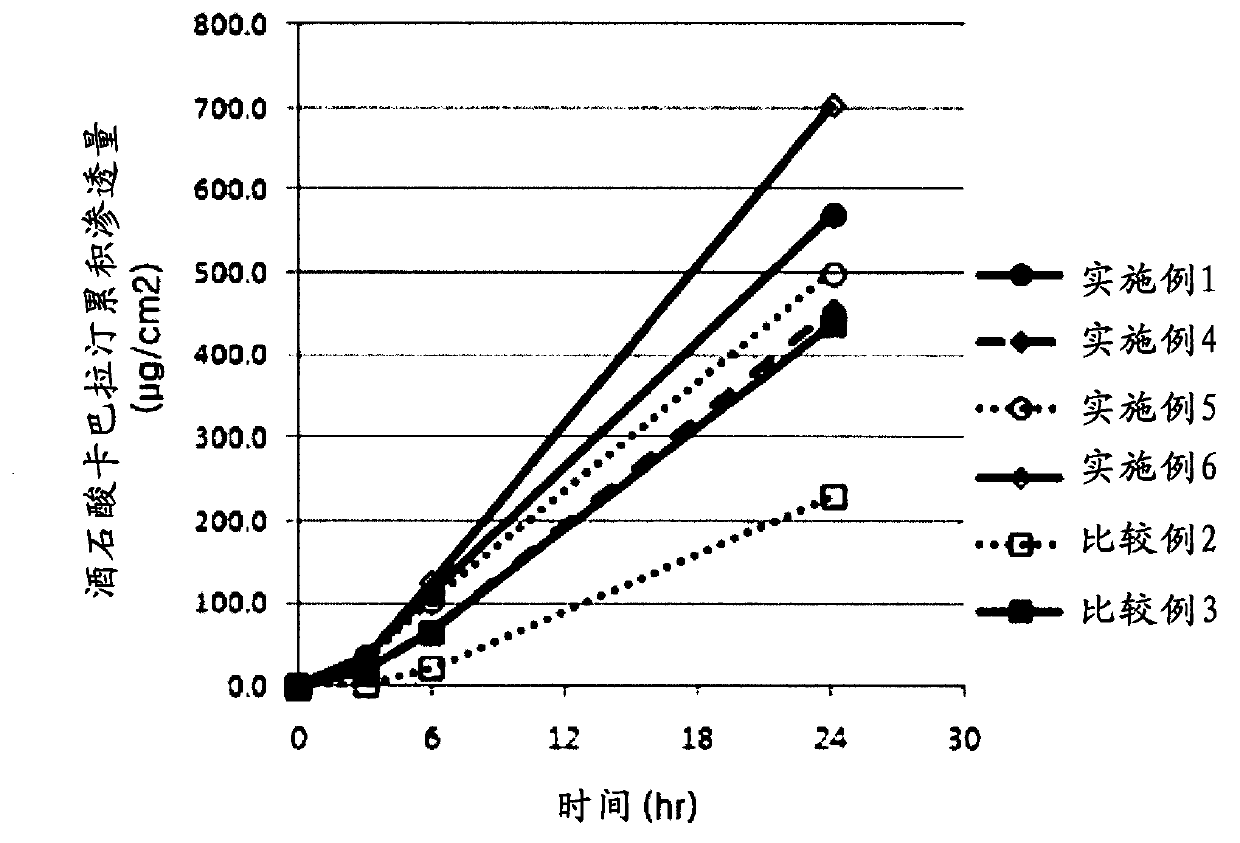
Examples
Embodiment 1-6
[0061] Examples 1-6: Transdermal preparations for the treatment of dementia containing rivastigmine as an active ingredient agent
[0062] Styrene-isoprene-styrene block copolymer (hereinafter referred to as "SIS") as the binder matrix, polybutene (PB2400, Daelim Petrochemical Company) as the plasticizer, cycloaliphatic Hydrocarbon resin (Kristalex F85, Eastman Chemical Company), aliphatic hydrocarbon resin (Escorez1401, ExxonMobil Chemical) and rosin ester resin (KE311, Arakawa Chemical) were dissolved in the solvent n-hexane to prepare binder solutions.
[0063] To the above adhesive solution was added rivastigmine and an optional skin penetration enhancer in the weight ratio of Table 1. The resulting homogeneous mixture was coated on a siliconized polyester release film, which was then dried at 90° C. for 5 minutes to prepare a sheet having the thickness specified in Table 1. The sheet was laminated with a polyester backing film, and cut to a prescribed size to obtain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com