Patch
一种贴剂、增粘剂的技术,应用在药物组合、油/脂肪/蜡非有效成分、神经系统疾病等方向,能够解决皮肤刺激性等问题,达到皮肤刺激性低、充分皮肤粘合性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~10
[0135] [Example 1-10] Preparation of patch containing rivastigmine
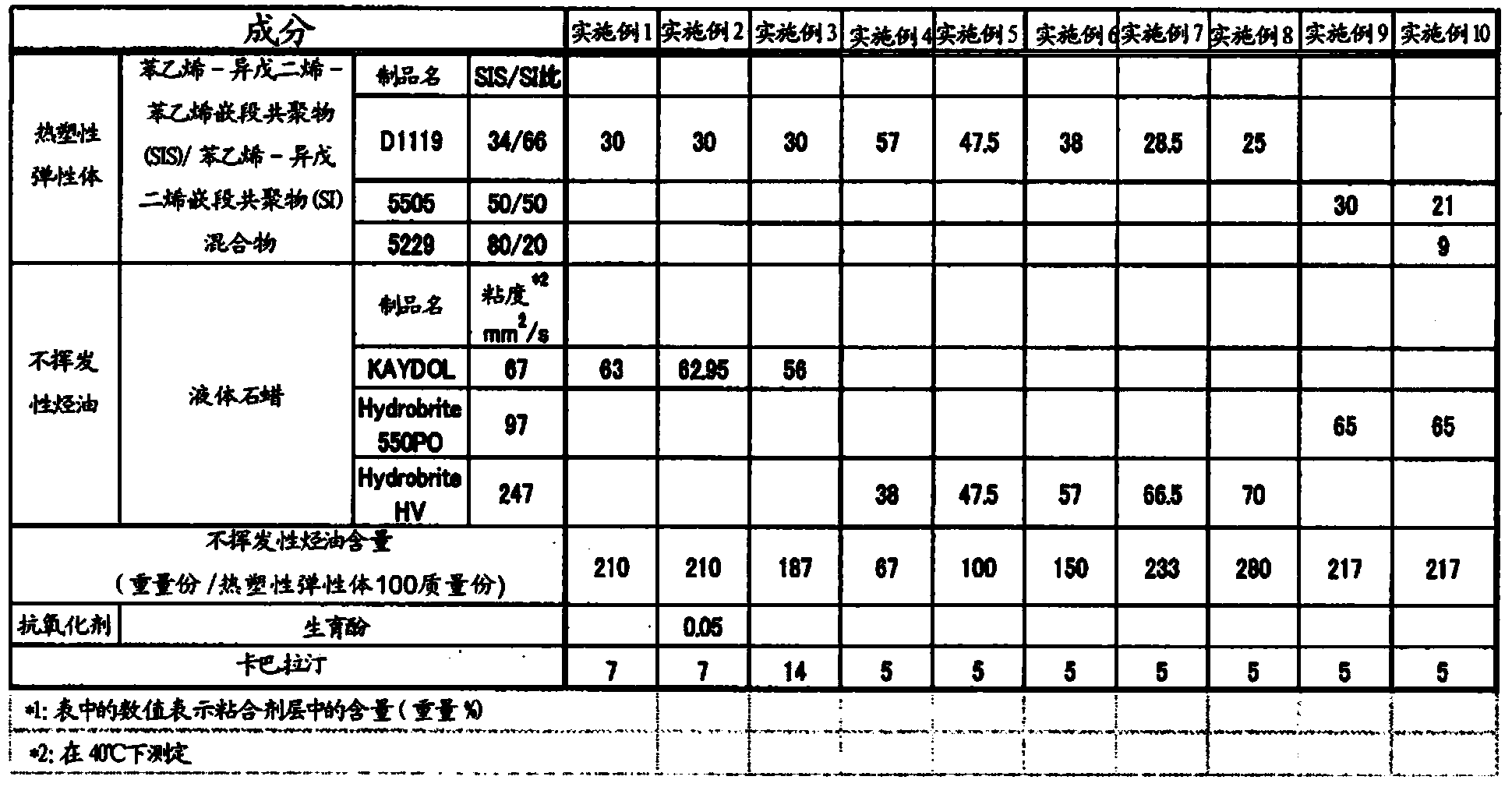
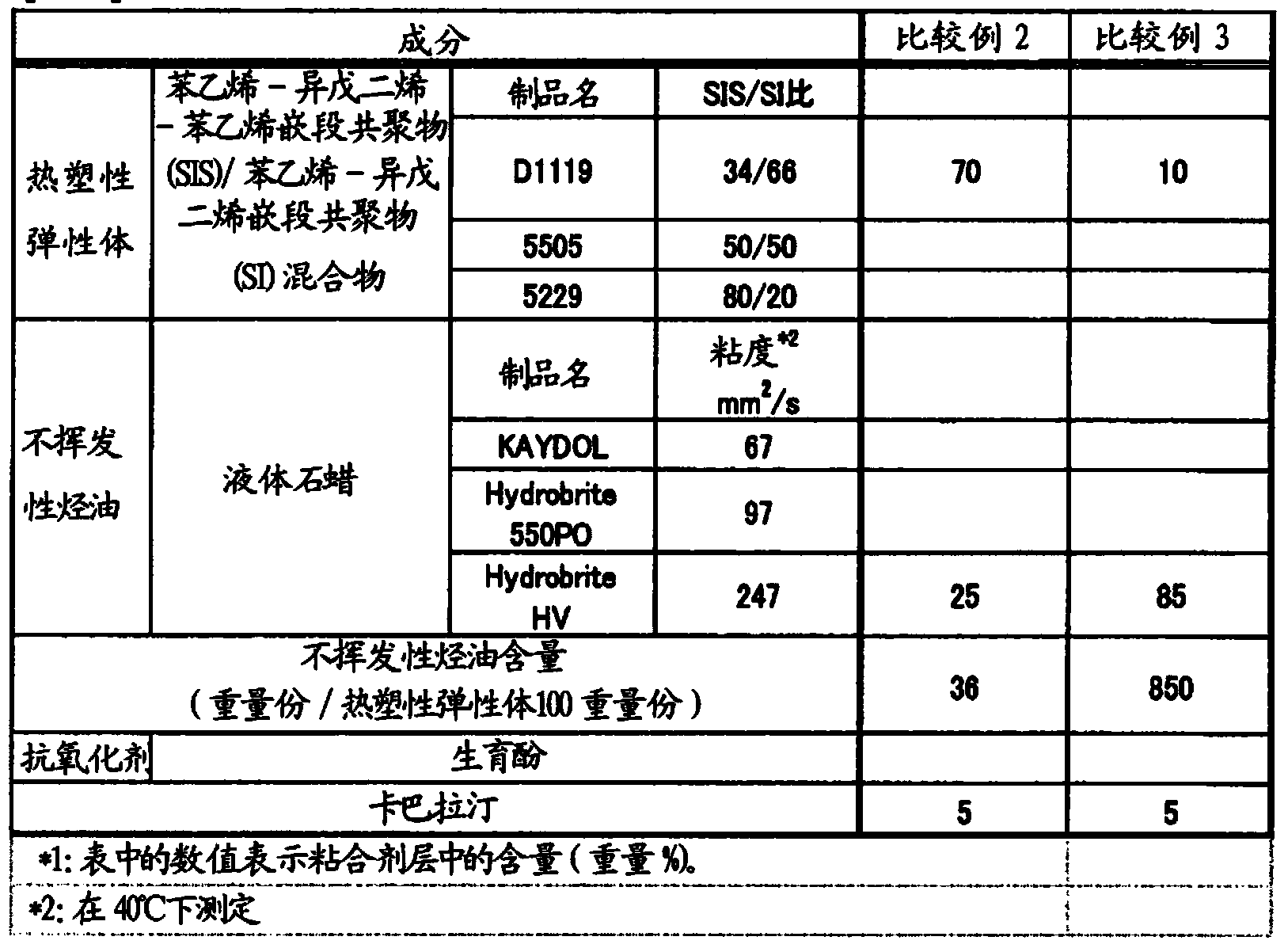
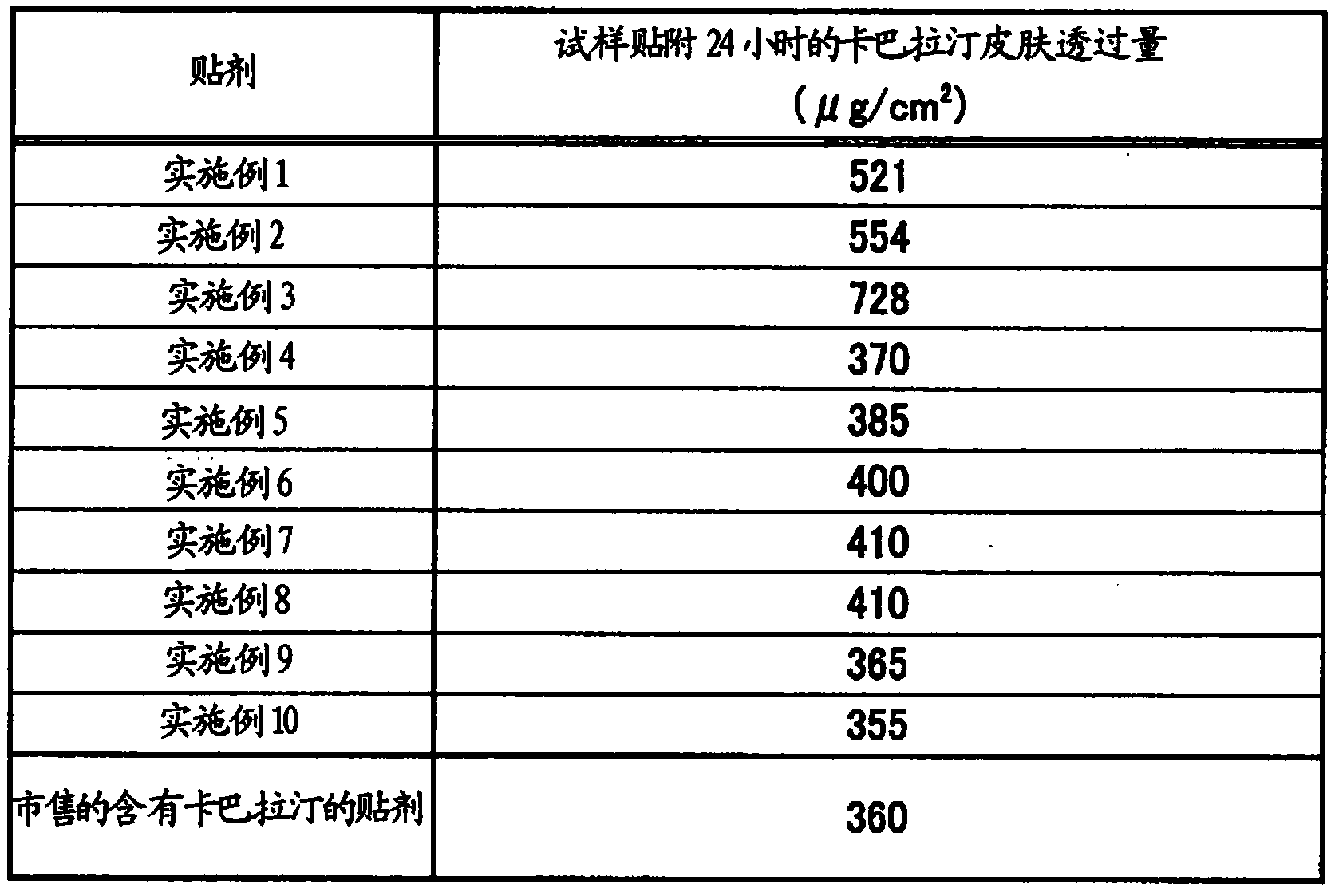
[0136]Each component constituting the adhesive layer was weighed according to the recipe shown in Table 1. First, a styrene-isoprene-styrene block copolymer (SIS) / styrene-isoprene block copolymer (SI) mixture ("KRAYTON D1119" manufactured by KRAYTON Corporation (weight average molecular weight: 207500 ), "JSR SIS5505" and "JSR SIS5229" manufactured by JSR Corporation) were dissolved in 230 parts by weight of toluene with respect to 100 parts by weight of the mixture. Liquid paraffin ("KAYDOL", "Hydrobrite 550PO", "Hydrobrite HV" manufactured by Sonneborn) rivastigmine was added to the above solution, mixed and stirred to prepare a coating solution for forming an adhesive layer.
[0137] In addition, the above-mentioned coating solution was coated on a silicone-treated polyethylene terephthalate (PET) film to prepare (release liner), and the rivastigmine content in the dried adhesive layer was adjusted to 1....
Embodiment 11
[0212] [Example 11] Preparation of patch containing rivastigmine
[0213] According to the prescription shown in Table 8, each composition that constitutes the drug storage layer was weighed. First, a styrene-isoprene-styrene block copolymer (SIS) / styrene-isoprene block copolymer (SI) mixture ("KRAYTON D1119" manufactured by KRAYTON Corporation (weight average molecular weight: 207500 )) was dissolved in 230 parts by weight of toluene with respect to 100 parts by weight of the mixture. Liquid paraffin ("Hydrobrite HV" manufactured by Sonneborn Co., Ltd.) and rivastigmine were added to the above solution and mixed with stirring to prepare a coating solution for forming a drug storage layer.
[0214] Prepared by coating the above-mentioned coating solution on a silicone-treated polyethylene terephthalate (PET) film (release liner), so that the rivastigmine content in the dried drug storage layer was 1.8 mg / cm 2 . After drying in an oven at 80° C. for 1 hour, a PET film (car...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com