Rivastigmine preparation suitable for industrial production
A compound and methylation technology, which is applied in the preparation of organic compounds, chemical instruments and methods, and the preparation of carbamic acid derivatives, etc., can solve the problems of large loss, low yield, no synthetic routes and examples, and achieve pollution Small size, easy access to raw materials, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of 3-((S)-1-((S)-1-phenethylamino) ethyl) phenylethyl (methyl) carbamate (compound 1)
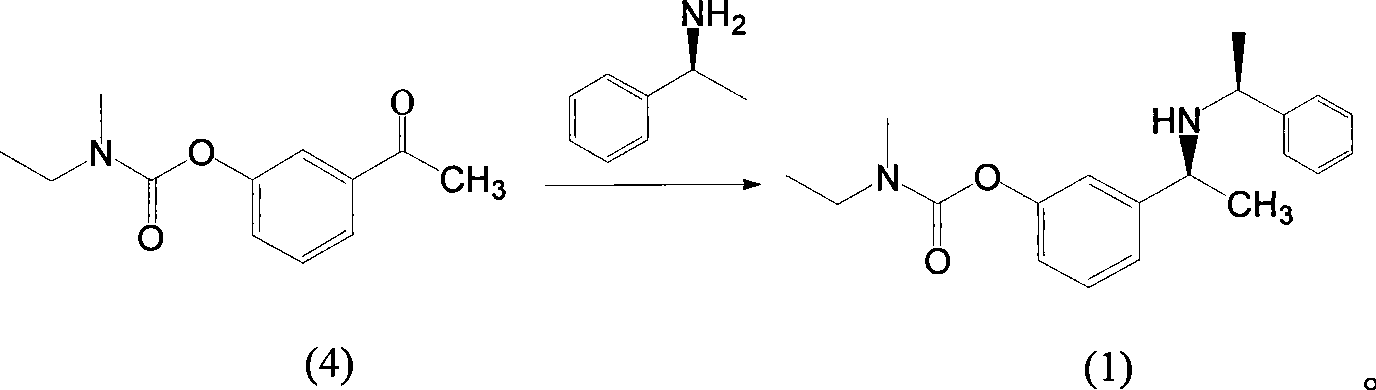
[0035] 10g (0.05mol) of (S)-1-phenethylamine, 5.5g (0.05mol) of (S)-1-phenethylamine, tetraisotitanate 19g (0.07mol) of propyl ester and 85ml of ethyl acetate were stirred and reacted at 30°C for 2 hours, 0.5g of 10% palladium carbon was added, hydrogen was passed, and the reaction was carried out at 65°C and 15atm for 15 hours, and the reaction of compound (4) was monitored by TLC. Cooling and suction filtration, add 100ml of 1N sodium hydroxide solution to the mother liquor, stir at room temperature for 1 hour, suction filtration, separate layers of the mother liquor, extract the water layer with 50ml of ethyl acetate twice, combine the ethyl acetate layers, and wash with saturated saline 80ml× Washed 3 times, dried with anhydrous sodium sulfate, then sucked and spin-dried to obtain 13.5 g of a yellow clear liquid with a yield of 92% and a purity of 98%.
Embodiment 2
[0036] Embodiment 2: Preparation of (S)-3-(1-aminoethyl) phenylethyl (methyl) carbamate (compound 2)
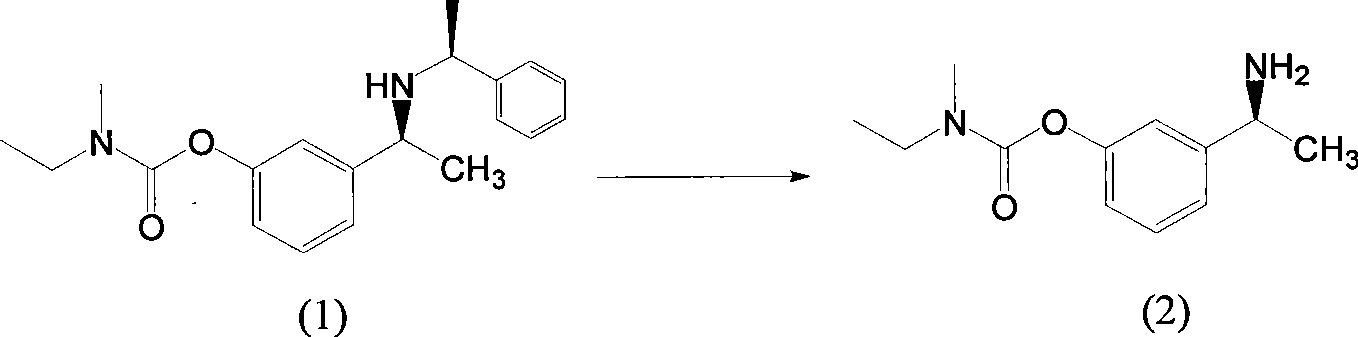
[0037] Add 10g (0.03mol) of compound (1) and 100ml of methanol into the reactor, add 0.5g of 10% palladium carbon, stir and pass hydrogen, react at 65°C and 15atm for 8 hours, TLC monitors that compound (1) has reacted completely, and cools Suction filtration, the mother liquor was spin-dried at 40°C, added 100ml of 10% potassium carbonate solution and stirred at room temperature for 1 hour, extracted with 50ml of ethyl acetate x 4 times, combined the ethyl acetate layers, washed with 50ml of saturated brine x 3 times, no After drying with sodium sulfate, the product was filtered and spin-dried to obtain 6.7 g of a yellow clear liquid with a yield of 98.5%.
Embodiment 3
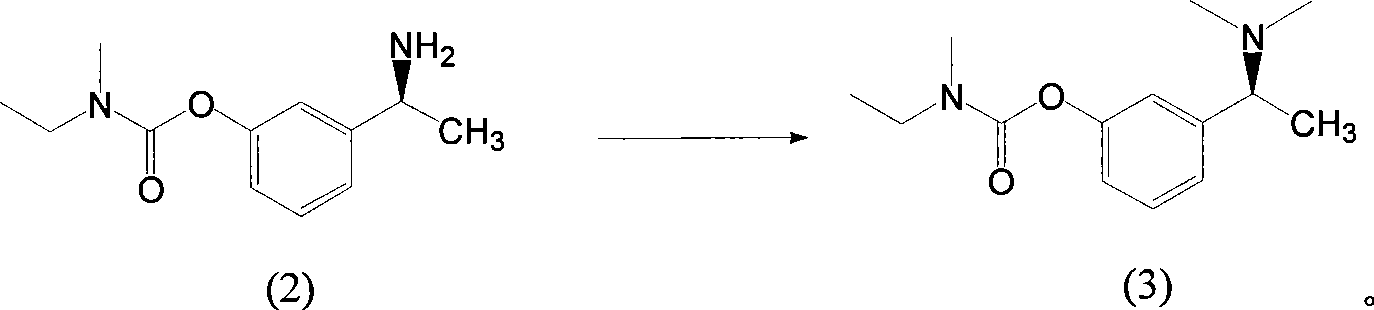
[0039] Add 10 g (0.03 mol) of compound (1) and 100 ml of methanol into the reactor, add 1 g of 10% palladium carbon, stir and pass hydrogen, and react for 96 hours at 35 ° C and 2 atm. TLC monitors that the compound (1) has reacted completely. Filter, spin dry the mother liquor at 40°C, add 100ml of 10% potassium carbonate solution and stir at room temperature for 1 hour, extract with 50ml of ethyl acetate x 4 times, combine ethyl acetate layers, wash with saturated brine 50ml x 3 times, anhydrous After drying over sodium sulfate, suction filtration and spin-drying gave 5.8 g of yellow clear liquid with a yield of 85.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com