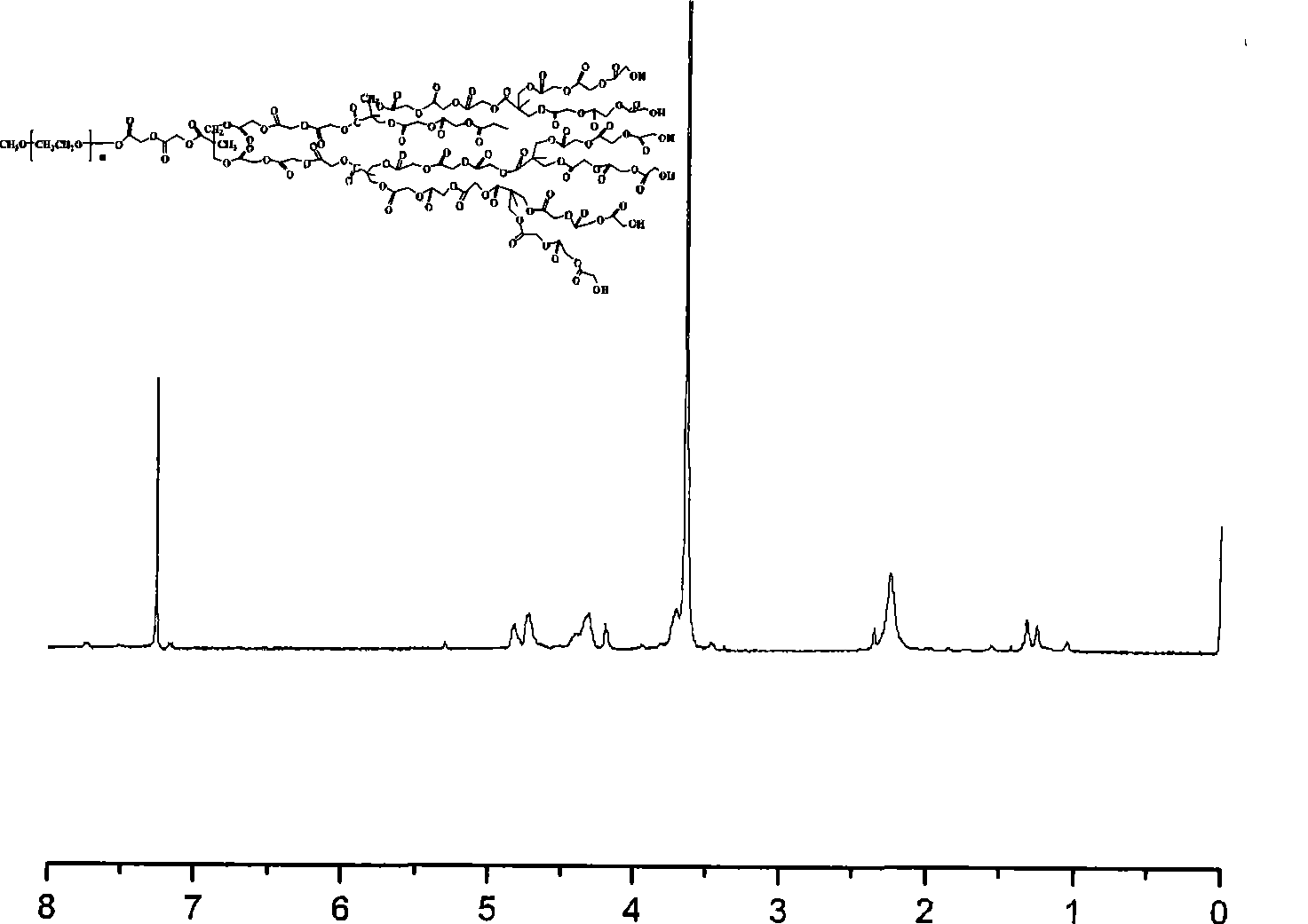
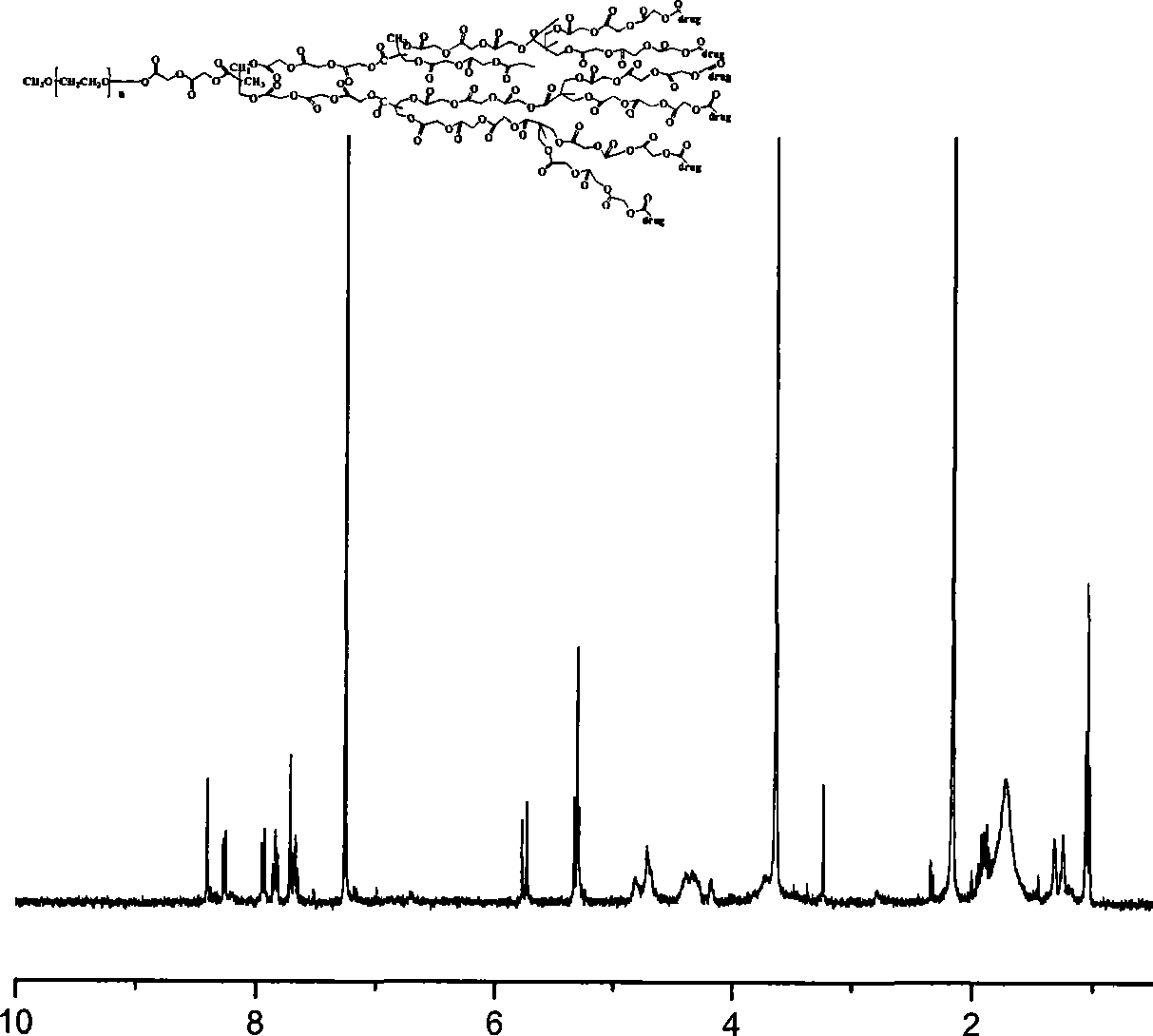
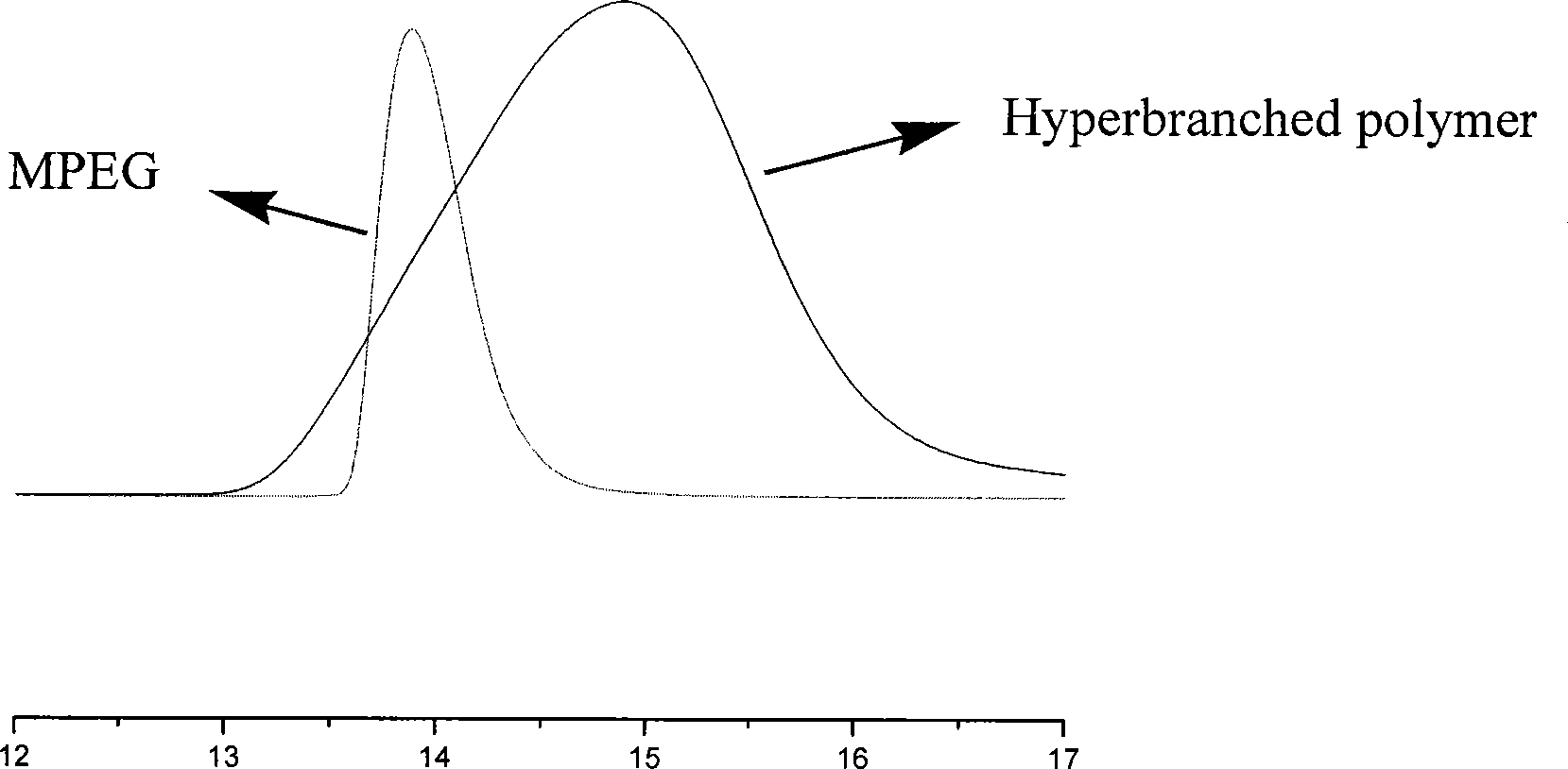
Anti-tumor prodrug using novel amphipathic hyperbranched polyesters as carrier and preparation method
An amphiphilic hyperbranched, anti-tumor technology, which is applied in the field of anti-tumor prodrugs and preparations, can solve the problems of difficult quality control of microspheres and microcapsules, unavoidable initial burst release, low production efficiency, etc., and achieve improved biocompatibility Sex, avoid toxic and side effects, enhance the effect of drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1) Add 9g of glycolic acid and 400ml of anhydrous ether into a 500ml single-necked flask, stir at room temperature until the glycolic acid is completely dissolved, then add catalyst, 4.56ml of tert-butyl bromide, and react at room temperature in the dark for 24 hours. Remove the precipitate in the reaction system by filtration, remove anhydrous ether by rotary evaporation, add dichloromethane to freeze and precipitate unreacted glycolic acid, extract with saturated aqueous solution of sodium bicarbonate, dissolve tert-butyl 2-hydroxyacetate in dichloromethane, and separate The organic phase and the aqueous phase were removed by rotary evaporation to remove dichloromethane to obtain 1.0 g of the product. The aqueous phase was adjusted to acidity with hydrochloric acid, then extracted with dichloromethane, and the methylene chloride was removed by rotary evaporation to obtain 1 g of 2-tert-butoxyacetic acid.
[0030] 2) In a 250ml single-necked flask, add 2g of glycolic ac...
Embodiment 2
[0036] 1) Add 11g of lactic acid and 400ml of anhydrous ether into a 500ml single-necked flask, stir at room temperature until the lactic acid is completely dissolved, then add catalyst, 4.56ml of tert-butyl bromide, and react at room temperature for 24 hours in the dark. Remove the precipitate by filtration, remove anhydrous ether by rotary evaporation, add dichloromethane to freeze and precipitate unreacted lactic acid, extract with saturated aqueous solution of sodium bicarbonate, dissolve the carboxyl group of lactic acid protected by tert-butyl group in dichloromethane, separate the organic phase and water phase, rotary evaporation to remove dichloromethane to obtain 1.0 g of the product, the aqueous phase was adjusted to acidity with hydrochloric acid, and then extracted with dichloromethane, rotary evaporation to remove dichloromethane to obtain 1 g of product tert-butyl-protected lactic acid hydroxyl.
[0037] 2) In a 250ml single-necked flask, add 2g of lactic acid wit...
Embodiment 3
[0040] Take 7 g of hyperbranched carboxylated polyester, 0.05 g of paclitaxel, 150 ml of tetrahydrofuran, 0.93 g of dicyclohexylcarbodiimide, and a catalytic amount of 4′4-dimethylaminopyridine, and react at room temperature for 24 hours. After the reaction, the urea salt was removed by filtration, and the tetrahydrofuran was concentrated. , slowly dropwise into anhydrous ether, let stand, and filter to obtain paclitaxel-loaded biodegradable amphiphilic hyperbranched polyester.
[0041] Other steps are the same as in Embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com