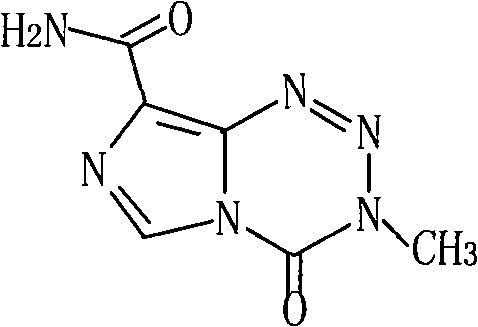
Temozolomide freeze-dried preparation
A technology of temozolomide and freeze-dried preparations, which is applied in the field of medicinal chemistry, can solve the problems of hidden dangers in the quality and safety of injection preparations, cumbersome operations, and long time, and achieve the effects of conforming to clinical medication habits, reducing toxic and side effects, and stabilizing quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: Preparation of temozolomide freeze-dried preparation for injection
[0028] (1) Preparation composition:
[0029] Temozolomide 250mg
[0030] L-Alanine 500mg
[0031] Polysorbate 80 400mg
[0032] Mannitol 1.5g
[0033] Sodium citrate 500mg
[0034] Hydrochloric acid 410mg
[0035] Water for injection Add water to make up to 100ml
[0036] (2) Preparation method:
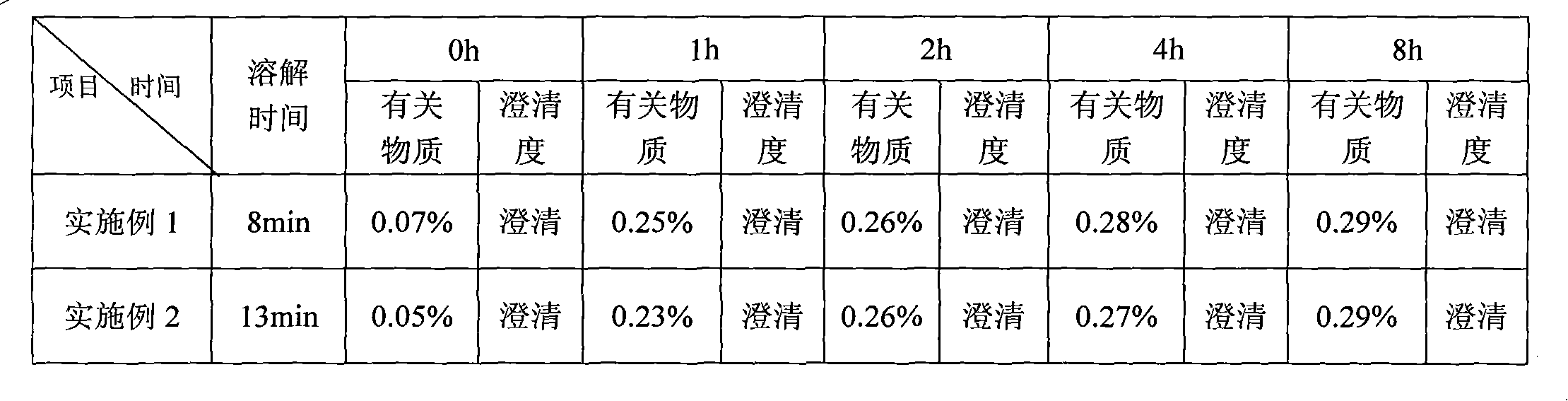
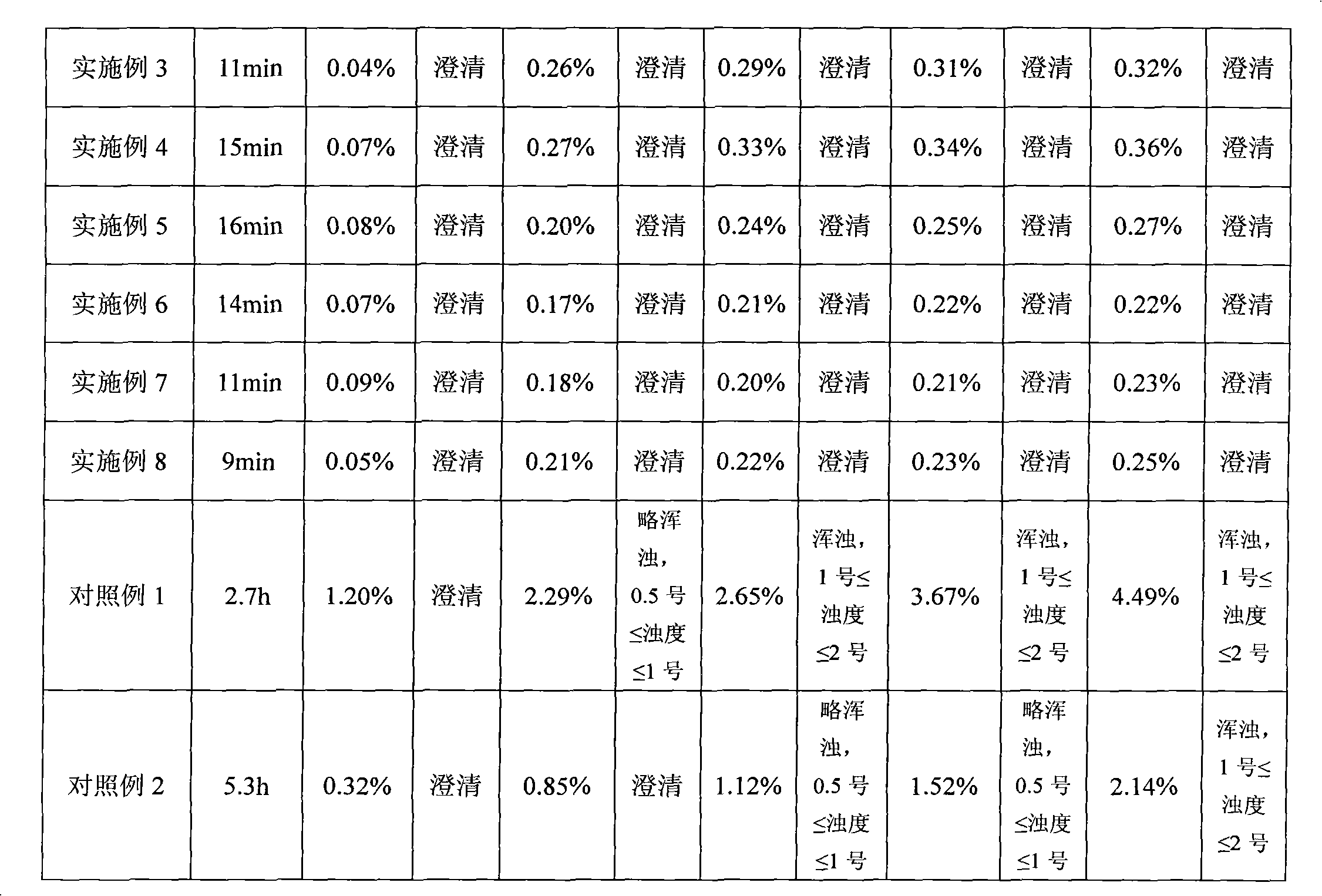
[0037] Weigh the above weight of L-alanine, polysorbate 80, mannitol, sodium citrate, hydrochloric acid, add 80ml of water for injection, stir for about 2 minutes, all the solids are dissolved and clear, add the prescribed amount of temozolomide, stir After about 8 minutes, all the solids were dissolved and clarified. Adjust the pH to 4.0 with hydrochloric acid solution or sodium hydroxide solution; add water for injection to make up the volume to 100ml, filter through a 0.22μm microporous membrane, take 20ml for room temperature solution placement test, and others The solution is divide...
Embodiment 2
[0062] Embodiment 2: Preparation of temozolomide freeze-dried preparation for injection
[0063] (1) Composition:
[0064] Temozolomide 100mg
[0065] L-Alanine Hydrochloride 100mg
[0066] Polysorbate 80 100mg
[0067] Mannitol 0.5g
[0068] Potassium Citrate 100mg
[0069] Hydrochloric acid 50mg
[0070] Water for injection Add water to make up to 100ml
[0071] (2) Preparation method:
[0072] Take by weighing the above-mentioned L-alanine hydrochloride, polysorbate 80, mannitol, sodium citrate, hydrochloric acid, add in 80ml water for injection, stir until all solids are dissolved and clear, add the temozolomide of prescription amount, stir until All the solids are dissolved and clarified, and the pH is adjusted to 6.0 with hydrochloric acid solution or sodium hydroxide solution; add water for injection to make up the volume to 100ml, pass through a 0.22μm microporous filter membrane to sterilize and filter, and divide into 30ml vials, each with 10ml The solution i...
Embodiment 3
[0074] Embodiment 3: Preparation of temozolomide freeze-dried preparation for injection
[0075] (1) Composition:
[0076] Temozolomide 1g
[0077] L-Leucine Hydrochloride 800mg
[0078] Polysorbate 80 600mg
[0079] Mannitol 2g
[0080] Citric acid 700mg
[0081] Hydrochloric acid 300mg
[0082] Water for injection Add water to make up to 100ml
[0083] (2) Preparation method:
[0084] Weigh the above weight of L-leucine hydrochloride, polysorbate 80, mannitol, citric acid, hydrochloric acid, add 80ml of water for injection, stir until the solids are all dissolved and clear, add the prescribed amount of temozolomide, and stir until solid Dissolve completely and clarify, adjust the pH to 6.0 with hydrochloric acid solution or sodium hydroxide solution; add water for injection to make up the volume to 100ml, pass through a 0.22μm microporous membrane filter to sterilize and filter, and divide into 30ml vials, each with 10ml of solution , put into a freeze-drying box, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com