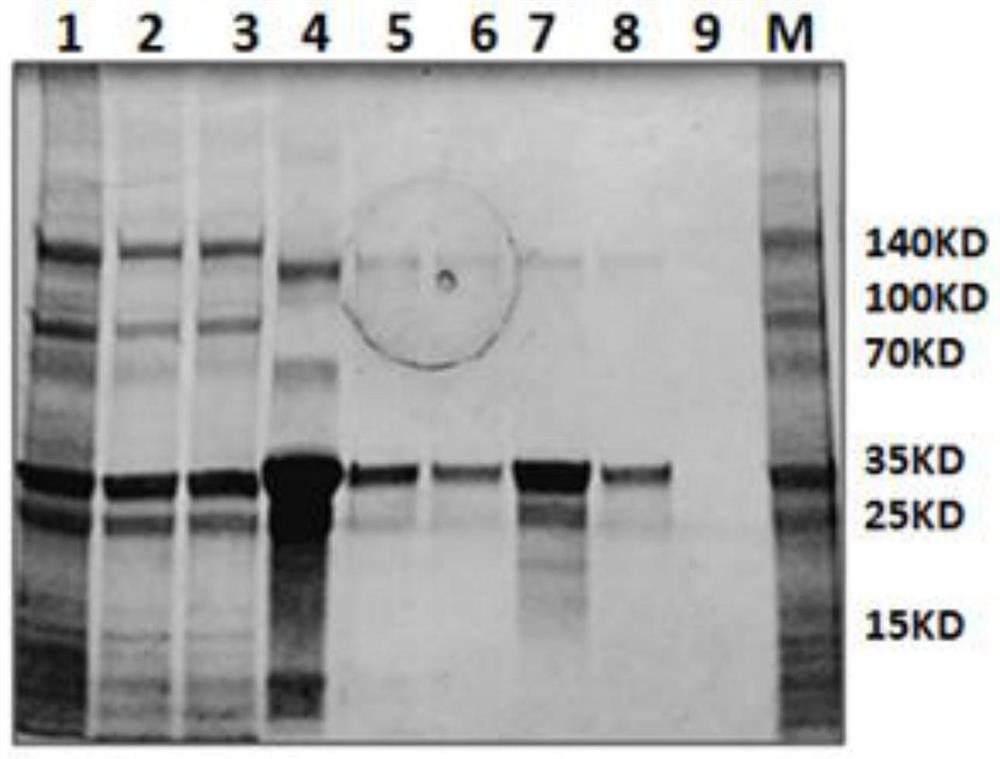
A method for large-scale production of high-purity porcine circovirus orf2 protein
A technology of porcine circovirus and ORF2, which is applied to the preparation method of peptides, biochemical equipment and methods, viruses, etc., can solve the problems of immune animal side effects and reduce vaccine efficacy, so as to solve side effects, improve processing efficiency, save cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 1. Antigen clarification pretreatment
[0048] Sterile chitosan was added to the porcine circovirus ORF2 protein culture solution in proportions of 1wt%, 5wt%, and 10wt%, respectively, after shaking well, it naturally settled at 4°C, and the supernatant was taken as the protein sample to be clarified.
[0049] Table 1 Evaluation of porcine circovirus ORF2 protein clarification pretreatment effect
[0050] sample Protein concentration (ug / ml) Miscellaneous protein removal rate Turbidity (FTU) Protein recovery Protein expression as is 11214 - 2000 - 1% Chitosan 5612 85.01% 1000 99.8% 5% Chitosan 4321 92.12% 300 99.1% 10% Chitosan 3123 93.50% 50 91%
[0051] Use A 280 Methods The protein concentration was detected and the effective target protein content was detected by double antibody sandwich ELISA. The experimental results are shown in Table 1. The results in Table 1 show that the addition of chitosan can sign...
Embodiment 2
[0053] 2. Hollow fiber column clarification process
[0054] 1 system pretreatment
[0055] 1.1 Install the 0.2μm, 0.45μm, and 0.65μm hollow fiber columns into the hollow fiber column control equipment respectively, connect the corresponding pipelines, and soak the hollow fiber columns with sterile injection water for 10 minutes after assembly.
[0056] 1.2 System Integrity Detection
[0057] The pressure hold method checks the integrity of the system.
[0058] 1.3 System processing
[0059] Cleaning and sterilization: Use sterile 0.5mol / L NaOH solution to sterilize the system for 30 minutes, then clean the system with sterile water for injection to remove residual alkali solution until the pH is 7.0;
[0060] 1.4 Detection of water flux
[0061] Use sterile water for injection to pass through at the specified pump speed, and calculate the water flux of the hollow fiber column at the corresponding temperature.
[0062] 1.5 hollow fiber column balance
[0063] Equilibrate...
Embodiment 3
[0070] Embodiment 3 ion exchange column purification process
[0071] 1 system pretreatment
[0072] 1.1 Pound the ion-exchange filler Capto SP ImpRes into the BPG300 / 500 column, and measure the column efficiency after the ion-exchange column is assembled.
[0073] 1.2 Treat the molecular sieve gel chromatography column with sterile 0.5mol / L NaOH for 2 column volumes (CV), then wash with sterile injection water to pH 7.5, and then equilibrate the ion exchange column with 20mmol / L Tris solution to reach the conductivity The column is consistent before and after (the conductivity before and after the column is 6.5~6.6ms / cm), the pH is stable at 7.0, and the UV 280 The baseline is stable.
[0074] 2 purification process
[0075] Load the protein clarified solution, set the linear flow rate of the sample to 30-100cm / h, control the pressure to less than 2.50bar, and control the sample volume to 70-80mg / ml BSA (the maximum loading capacity of the filler is 95mg / ml BSA) . After ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com